
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
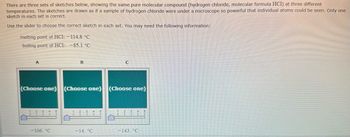
Transcribed Image Text:There are three sets of sketches below, showing the same pure molecular compound (hydrogen chloride, molecular formula HCl) at three different
temperatures. The sketches are drawn as if a sample of hydrogen chloride were under a microscope so powerful that individual atoms could be seen. Only one
sketch in each set is correct.
Use the slider to choose the correct sketch in each set. You may need the following information:
melting point of HCI: -114.8 °C
boiling point of HC1: -85.1 °C
A
B
C
(Choose one) (Choose one) (Choose one)
3
-106. °C
-14. °C
143. °C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Maple syrup sap is 3% sugar (sucrose) and 97% water bymass. Maple syrup is produced by heating the sap toevaporate a certain amount of the water. (a) Describe what happens to the composition and boilingpoint of the solution as evaporation takes place. (b) A rule of thumb among maple syrup producers is thatthe finished syrup should boil about 4 C higher than theoriginal sap being boiled. Explain the chemistry behindthis guideline. (c) If the finished product boils 4 C higher than the originalsap, calculate the concentration of sugar in the finalproduct. Assume that sugar is the only solute and theoperation is done at 1 atm pressure.arrow_forwardDefine the normal boiling point of water. Why does a sample of boiling water remain at the same temperature until all the water has been boiled? Define the normal freezing point of water. Sketch a representation of a heating/cooling curve for water, marking clearly the normal freezing and boiling points.arrow_forwardCooking A cook prepares a solution for boiling by adding12.5 g of NaCl to a pot holding 0.750 L of water. Atwhat temperature should the solution in the pot boil?Use Table 14.5 for needed data.arrow_forward
- In a mountainous location, the boiling point of pure water is found to be 95C. How many grams of sodium chloride must be added to 1 kg of water to bring the boiling point back to 100C? Assume that i = 2.arrow_forwardConsider two hypothetical pure substances, AB(s) and XY(s). When equal molar amounts of these substances are placed in separate 500-mL samples of water, they undergo the following reactions: AB(s)A+(aq)+B(aq)XY(s)XY(aq) a Which solution would you expect to have the lower boiling point? Why? b Would you expect the vapor pressures of the two solutions to be equal? If not, which one would you expect to have the higher vapor pressure? c Describe a procedure that would make the two solutions have the same boiling point. d If you took 250 mL of the AB(aq) solution prepared above, would it have the same boiling point as the original solution? Be sure to explain your answer. e The container of XY(aq) is left out on the bench top for several days, which allows some of the water to evaporate from the solution. How would the melting point of this solution compare to the melting point of the original solution?arrow_forwardHeat is released when some solutions form; heat is absorbed when other solutions form. Provide a molecular explanation for the difference between these two types of spontaneous processes.arrow_forward
- A 0.500-g sample of KCl is added to 50.0 g of water in a calorimeter (Figure 5.12). If the temperature decreases by 1.05 °C, what is the approximate amount of heat involved in the dissolution of the KCl, assuming the specific heat of the resulting solution is 4.18 J/g °C? Is the reaction exothermic or endothermic?arrow_forwardou place hot metal into a beaker of cold water. ol type='a'> Eventually what is true about the temperature of the metal compared to that of the water? Explain why this is true. i>Label this process as endothermic or exothermic if we consider the system to be the metal. Explain. the water. Explain.arrow_forwardIf a substance has a positive enthalpy of solution, which would likely cause more of it to dissolve, hot solvent or cold solvent? Explain.arrow_forward
- Describe, on both a microscopic and a macroscopic basis, what happens to a sample of water as it is cooled from room temperture to 50Cbelow its normal freezing point.arrow_forwardConvert the units of Henrys law constant for CO2, in table 7.2, to units of, mmHg, atm, and bar. In which cases does the numerical value of the constant change?arrow_forward8.52 Rank the following hydrocarbons in order of increasing vapor pressure: C2H6,C10H22,CH4,C7H16,C22H46 .arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
