
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
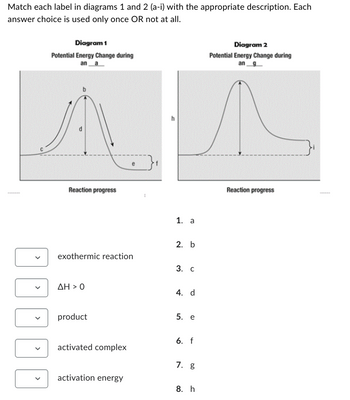
Transcribed Image Text:Match each label in diagrams 1 and 2 (a-i) with the appropriate description. Each
answer choice is used only once OR not at all.
Diagram 1
Potential Energy Change during
ana
Reaction progress
exothermic reaction
AH > 0
product
activated complex
activation energy
1. a
2. b
3. C
4. d
5. e
6. f
7. g
8. h
Diagram 2
Potential Energy Change during
an
Reaction progress
******
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- d' II ALEKS- Courtney Wh x New Tab X Lab 11-Atomic Finge G pericdic table-Goog x ALEKS rate Ex X lab 10 empirical form www.awu.aleks.com/alekscgi/x/lsl.exe/1o_u-lgNslkr7j8P3jH-lijulplKKWv4bwenmyrLYoBHbAAsJtmzj53GErnx_viTdHbIGMF9jvgkSu_nrizREmdQXHPJbMzq0mOrXJcuonZITQhB66q?1oBw7QYjb Courtne 45 O CHEMICAL REACTIONS Theoretical yield of chemical reactions reacts with gaseous oxygen gas (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,0). What is the (CH,(CH.),CH) Gaseous butane theoretical yield of carbon dioxide formed from the reaction of 5.81 g of butane and 28.5 g of oxygen gas? Be sure your answer has the correct number of significant digits in it. Terms of Use Privacy Center Accessibility ©2022 McGraw Hill LLC. All Rights Reserved. Check Explanation 1I0 -> $4 4. 8. 23 51 3. k 00 S a. u q ctrl 145 altarrow_forwardHelp plsarrow_forwardMicrosoft Teams Q Search ... 25 Assignment on matching phrases on classification_ units.docx Close Activity File Home Insert Layout References Review View Help O Tell me what you want to do 'Editing v O Comments A Catch up Chat Calibri (Body) v 11 A В I U Av A A Styles v O Find v Reuse Files O Dictate v E Editor : Designer ... ... MATCHING OF WORDS OR PHRASES Teams 1. Assignments 1. Alloys a) example of a derived unit 2. 2H b) allotropes of oxygen Calendar 3. Mixtures c) two or more different elements bonded 4. Smallest unit of a compound d) centi ... 5. Compound e) a qualitative statement 6. 02 and 03 f) kilo 7. Smallest unit of a compound g) a unit of measurement 8. One thousandth of a unit h) a quantitative statement 9. Teaspoon i) components separable by physical means 10. H2 j) homogeneous mixtures of metals 11. Density k) milli 12. A person is tall I) a molecule of hydrogen 13. one thousand of something m) atom 14. one hundredth n) two atoms of hydrogen 15. Somebody weighs 150…arrow_forward
- envellum.ecollege.com/course.html?courseld=15432274&HepID=2b3e48e6520860bfd5591538a4a5a27b#10001 Search... Ael. AOL Video- Serving the best vi.. nline Sh... TripAdvisor 33 of 40 I Review | Constants | Periodic Table Learning Goal: To become familiar with the concept and calculations of specific heat. Part A Specific heat (which can be represented as SH, Cs, sp. ht., or a number of other possibilities) is defined as the amount of energy needed to raise the temperature of 1 g of a substance by 1 °C. For example, 0.0920 cal is enough energy to raise 1 g of copper from 21.0 °C to 22.0 °C. Therefore, the specific heat of copper is 0.0920 cal/(g - °C). How much heat energy is required to raise the temperature of 0.360 kg of copper from 23.0 °C to 60.0 °C? The specific heat of copper is 0.0920 cal/(g- °C). Express your answer with the appropriate units. > View Available Hint(s) Using SH for specific heat, the formula for calculating specific heat is HA Value Units heat heat = SH = massxAT P…arrow_forwardK Haylee Scott - cp Zoom meeting lin com/web/viewer.html?state%=D%7B'ids %3A%5B"1zOflgo1GoQFrdviJ6PWx8L3h2j4uav5n"%5D%2C"action"%3A'open C Clever | Portal A Classroom https://sso.theleam. M Oro Grande Elemen. W Yearbook Aven ue Period 5 Scien. Haylee Scott - cprr061.pdf Building Vocabulary From the list below, choose the term that best completes each sentence. matter physical change endothermic reaction chemical change chemistry precipitate exothermic reaction 7. Any change that alters a substance without changing it into another substance is a(n) 8. is anything that has mass and takes up space. 9. A reaction that releases energy in the form of heat is called a(n) 10. A(n) absorbed. 11. A chemical reaction is also referred to as a(n) is a reaction in which energy is 12. A(n) a chemical reaction. is a solid formed from a solution during 13. is the study of the properties of matter and how matter changes. OPearson Education, Inc., publishing as Pearson Prentice Hall. All rights reserved…arrow_forwardIintul N F CI Br Save and Close MacBook Air 吕口 F3 000 F4 F5 F6 F7 F8 F9 F10 %23 % & 4 5 6 7 8 9 Y U Rarrow_forward
- Part carrow_forwardCUTICLE OIL O Microsoft W final E.A 1. G fahrenheit x 9 Learning M X O Schoology x O ch 9 key A mukilteo.schoology.com/common-assessment-delivery/start/4592487484?action on.. Bb Molar Mie O Periodic x 3-6, 6-5, & 6-6 Test 6 of 10 © 29 POSSIBLE POINTS: 2 What is the mass of a 5.521 mole sample of MgCl2? O 17.26 g O 100.7 g O 525.7 g O 256.1 g 4. 6 8 9 10 Lenovo DI 23 $ 7 4 t r e ーの づarrow_forwardWhich of the following are important sources of error in cartographic products? Select one: OA. Generalization OB. Digitization OC. Annotation OD. Fuzzy boundariesarrow_forward
- Chrome File Edit View History Bookmarks People Tab Window Help Hcc Dashbc x E Buy Es: x G find so x © Periodi x A ALEKS X HUc Chapte x E New m x G conver X 不→ C A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNsIkr7j8P3jH-IQiHqRdYV_6Ux63SypJXz0Coxvwqgg4JkWI7FgD9QGpr.. O GASES O OC D Jacqueline v Using Avogadro's Law Hydrogen gas and nitrogen gas react to form ammonia gas. What volume of ammonia would be produced by this reaction if 7.5 m of nitrogen were consumed? Also, be sure your answer has a unit symbol, and is rounded to 2 significant digits. 圖 中 ロ alo oloarrow_forwardAnswer question number 7 on the attached documentarrow_forwardes-2021S2 Allison.E CHEM X 2.ga.us/d21/Ims/quizzing/user/attempt/quiz_start_frame_auto.d2l?ou=2593077&isprv=&drc%3D1&qi=2478023&cfql=0&dnb%3D08fi lancer O Khan Academy E DeltaMath musictheory.net - E. Ca Desmos | Scientific. g Final Exam Ciara Smith: Attempt 1 D View hint for Question 18 Question 19 (3 points) Explain how OCompound A and Compound B will dissolve in water using the correct vocabulary including solvation, dissociatic n, ionic, and covalent. O Mg Cl CH Compound A Compound B hp 二arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY