
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:bAnswered: 101 Chem 101 b X G how many kilograms in a p x
(274) Ésta Es Tu Canció
101 Chem 101
X
Home
X
app.101edu.co
Oregon Scholarship....
Unofficial Transcript...
myClackamas Login
Document Require...
Apps WLogon
Welcome to the OS...
Home - FAFSA on t...
The National Societ...
>
Submit
Question 19 of 20
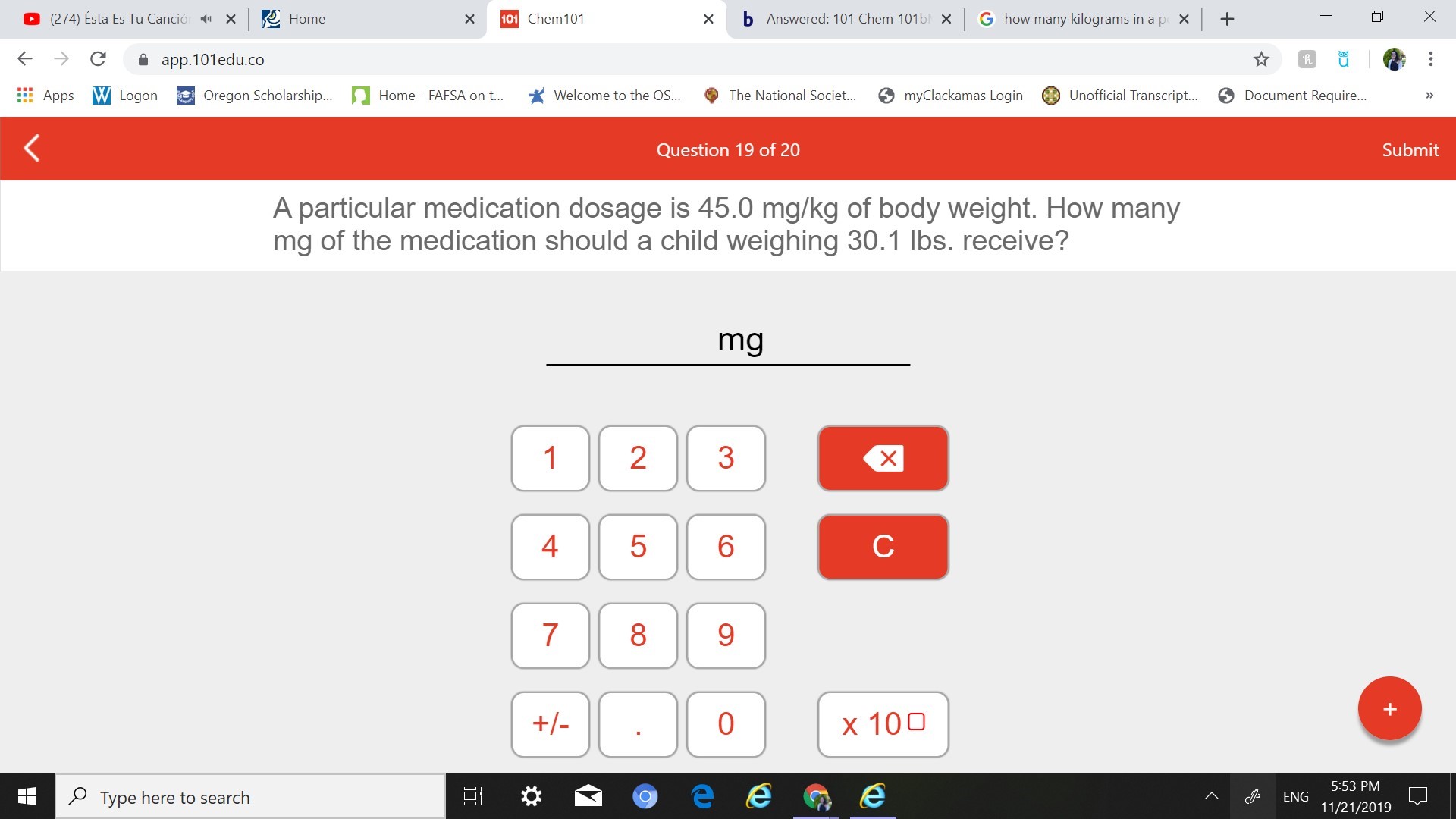
A particular medication dosage is 45.0 mg/kg of body weight. How many
mg of the medication should a child weighing 30.1 lbs. receive?
mg
1
2
3
X
5
6
C
7
8
+-
0
x 100
5:53 PM
Type here to search
ENG
11/21/2019
+
LO
4t
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Can you please help with the table? Thanksarrow_forwardWhat is the empirical formula of copper chloride for this lab?arrow_forward92% D Sun 3:04 PM OneLogin B McGraw-Hill Can O Question 1- 8.4 M MHE Reader B McGraw-Hill Cam x A ALEKS - Esther C x -> A www-awa.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IBcn9hvCfbYq_fi3Zsn8H2oW_5PTMiOacVC-TPIQJ5aqZ8Tg8iSQs6Rzj49leNgLwH_Jfre.. ☆ -pps M Gmail O YouTube O Maps BC Broward College |.. O Sample Source An.. O New Tab G What does Duckw. E Untitled documen. B Reading List O KINETICS AND EQUILIBRIUM Using reactant reaction order to predict changes in initial rate Esther A certain reaction is first order in H, and first order in I,. Use this information to complete the table below. Be sure each of your answer entries has the correct number of significant digits. [4] [1] initial rate of reaction 1.25 M 1.39 M 15.0 M/s do 0.431 M 1.39 M 2.06 M 0.844 M Check Explanation O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use I Privacy | Accessibility 642 4 MacBook Airarrow_forward
- CH6_Chem103 - Kenai Peninsu X + to.mheducation.com/ext/map/index.html?_con=con&external_browser=0&launchUrl=https%253A%252F%252Fclasses.alaska.edu%252Fwebapps... 054 520 2 X 9 part 2 Homework i 47:54 J bok 50 O int rint erences C 125 aw 11 :0 FI 2 Question 11 - Chapter 9 part 2 X Determine the volume (in mL) of a 13.3 MHC2H3O2 stock solution must be used to prepare 200.0 mL of a solution that is 0.719 M HC2H302. F2 W mL HC2H302 # 3 00 20 F3 E $ 4 000 000 F4 R % 5 F5 U 8 DII F8 1 ( 9 DD F9 0 3 F10 Help P Savarrow_forward%3D 1eg= lo00g 4. Perform each conversion. 3.55 kg to grams un. a)arrow_forwardA bbhosted.cuny.edu/webapps/assessment/take/take.jsp?course_assessment_id=_1... R Paused E Apps G Gmail O YouTube O Maps Home Take Test: Test #1 * Question Completion Status: A MOving to the next question prevents cnanges to tnis answer. Question 4 of 20 > Question 4 4 points Save Answer What is the density of a solid sample that increased the volume of 25.2 mL of water to 34.9 mL when placed in the graduated cylinder and weighs 13.34 g? O A. 0.52 g/mL O B. 0.38 g/mL OC. 1.37 g/mL O D.0.73 g/mL A Moving to the next question prevents changes to this answer. Question 4 of 20 13,846 MAR étv S esc F2 F3 F4 F5 F6 F7 @ %23 24 2 & Q W E R Y IIarrow_forward
- 1PrqdNDx-hBYyzKo-xfiM9-Jlq7EAaWe3GBt t3U3jDo/edit ulator O A Land of Permane... it was seconds ago - UA E- EE 2 Al + 3 H,SO, 6. How many grams of hydrogen gas are produced with 0.S8 moles of aluminum sulfate? 3 H, + Al,(SO); 0.88 moles Al (SO), 3 moles H. 6 g H. 0.88 moles Al (SO), 1 mole H.arrow_forwardMyCSU - Columbus State Univer. x | № Inbox - bailes_amber@columbu: x D2L Homepage - Columbus State Un X - со A ALEKS-Amber Bail www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lJgXZp57itWHhRgilODc5MqvhZbKYx2-U-037007TYd Gmail YouTube Maps MyCSU - Columbus... Homepage - Georg... Microsoft Office Ho... B Lesson 2 Disc O CHEMICAL REACTIONS = Solving for a reactant using a chemical equation Ammonia (NH3) chemically reacts with oxygen gas (0₂) to produce nitric oxide (NO) and water (1₂0). What mass of ammonia is consumed by the reaction of 9.9 g of oxygen gas? Be sure your answer has the correct number of significant digits. x10 g ANAKKALE X S ? Email 4 Jessy Vseforestainty a jedan den so $45******arrow_forward2 3 4 -5 g . Use this information to calculate K.n for PbCO2. The solubility of PbCO, in water at 25 °C is measured to be 7.3 × 10 sp Round your answer to 2 significant digits. Continue O 2022 McGraw Hill LLC. All Rights Reserved. Ter II 20arrow_forward
- Home 101 Chem 101 My Questions bartleby X (274) Banda Carnaval - Sueñ X X X app.101edu.co Unofficial Transcript... Oregon Scholarship.... Welcome to the OS... myClackamas Login Document Require... Apps WLogon Home FAFSA on t... The National Societ... > Submit Question 5 of 20 If 450 g of magnesium hydroxide is dissolved in water to make 6.5 L of solution, what is the concentration in mM? mM 2 1 4 5 6 с 7 8 +- 0 x 100 5:05 PM Type here to search о ENG 11/21/2019 LOarrow_forwardSmartwork5 Gen Chem X ttps://ncia.wwnorton.com/78450 SOUME E 02/02/21 2 Learning Objective: 1.J Set up dimensional analysis conversions. Question 1st attempt Do not include spaces in your answer. Fill in the appropriate unit conversions. (Report the molar mass of KCI using three sig. figs.) x x. =. | +, | log. cos. 8. mol 5.00 g KC1 1 mol KC1 100. mL 1L KC1 <13 pe here to search 99+ C F5 F3 F4 F6 F7 F8arrow_forwardEmerson M Cremistr pdated late workE a educate.lindsay.k12.ca.us/iFrame.aspx?iCtri=STUDENT BASE HOME CONTROL O Empower 2021 O Ermpower 2021 pokmarks DE IXL Classes O Google Slides O Empower 2020 A Google Docs t Lindsay High School Physics Safety & Ski. E Guided pract 2-1 googlegalaxysclence.com Li Be 1-0 1-5 2-0 Al 2.5 3-0 3-5 Na Mg 1-2 Si S CI 0-9 1-5 1-8 Ge 2-1 2-5 3-0 K Ca Ga 1-6 As Se Br 0-8 1-0 1-8 2-0 2-4 Te 2-8 Rb Sr In Sn Sb 0-8 1-0 1-7 1-8 Pb 1-9 2-1 2-5 Cs 0-7 Ba TI Bi Po At 2-2 Review the chart above. It is showing how much energy it takes to remove an electron from each of those types of atoms. Why would 0.9 1-8 1-9 1-9 2-0 it be so high in the top right comer and so low in the lower len comer? It would be high in the top right coner because these electrons are closer to the nucleus and held more strongly, so they will take O A. more energy to remove. And the opposite is true for the lower left, farther from the nucleus and so not held as strongly, requiring less energy to…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY