
Chemistry: Matter and Change
1st Edition
ISBN: 9780078746376
Author: Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher: Glencoe/McGraw-Hill School Pub Co
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Many
a.
Carboxyl
b.
Amino
c.
amide
d.
methyl
e.
Sulfhydryl
Expert Solution
arrow_forward
Step 1
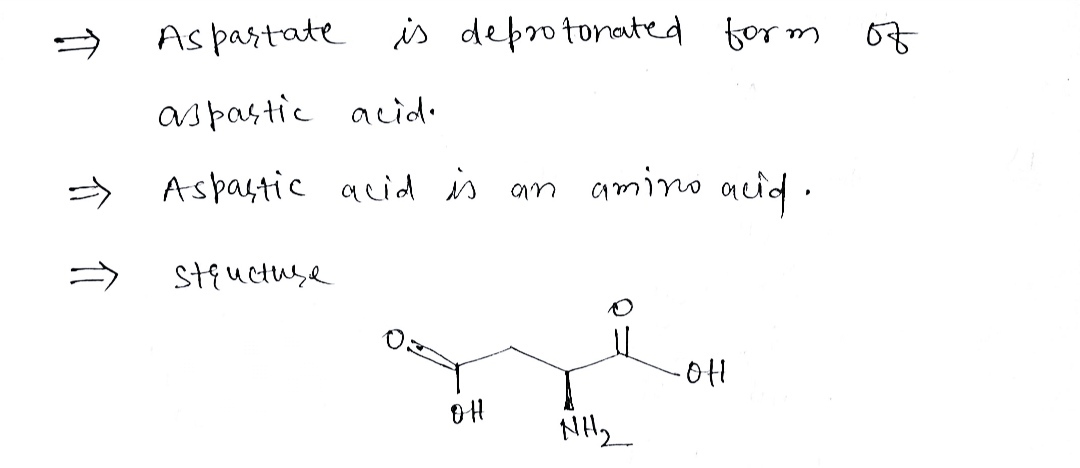
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (a) How many tripeptides can be made from glycine, alanine, and leucine, using each amino acid only once per tripeptide? (b) Write the structural formulas of these tripeptides and name them in the shorthand abbreviation used for showing amino acid sequences.arrow_forwardWhich structure corresponds to CH2=CHCH3?arrow_forwardWhat functional groups are found in all amino acids? How many different amino acids are found in naturally occurring proteins?arrow_forward
- what is the importance of isomers in biological systems?arrow_forwardThe functional group present in proteins is aldehyde a b amide hydroxyl C d. ketonearrow_forwardWhich compound has the highest boiling point? A. methoxyethane B. trimethylamine C. ethylmethylamine D. propylamine E. isopropylaminearrow_forward
- Paraformaldehyde is a polymer of A. acetone B. paraform C. formaldehyde D. acetaldehydearrow_forwardClassify the functional groups in the following steroid (choose all that apply).CHOOSE ALL THAT APPLY a. tertiary alcohol b. amide c. alkene d. secondary alcohol e. alkyne f. primary alcohol g. carboxylic acid h. aldehyde i. ester j. ketonearrow_forwardWhat are fats and oils? a. ketones formed of two or more esters b. any large molecular weight organic compound that has been saturated c. esters formed from glycerol and three carboxylic acids d. polymers of repeating aromatic hydrocarbonsarrow_forward
- A saturated fatty acid is a. a fatty acid with cis-carbon-carbon double bonds b. a fatty acid which has all single carbon-carbon bonds joining carbon atoms c. a fatty acid with a carbon chain which has just one carbon-carbon double bond d. a fatty acid chain in which a carbon chain has two or more carbon-carbon double bondsarrow_forwardThe dehydration of an alcohol produces which organic functional group and steam? a. alkyne b. aldehyde c. alkene d. ketone e. carboxylic acid and an alcoholarrow_forwardMatch the structures with corresponding functional groups: a. ester b. carboxylic acid c. alkyne d. alkene e. alcohol f. aromatic compound g. ketone h. alkane i. aldehyde j. aminearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div  ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
