
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Classify the
CHOOSE ALL THAT APPLY
a. tertiary alcohol
b. amide
c. alkene
d. secondary alcohol
e. alkyne
f. primary alcohol
g. carboxylic acid
h. aldehyde
i. ester
j. ketone
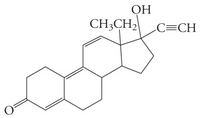
Transcribed Image Text:ОН
CH;CH,
C=CH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please complete the following question. There is no need to provide an explaination. 1arrow_forward1. The following molecule contains the functional groups A. secondary amine, secondary alcohol, ketone, carboxylic ester B. amide, primary alcohol, aldehyde, carboxylic acid C. secondary amine, two secondary alcohols, and two ketones D. primary amine, secondary alcohol, ketone, and carboxylic acid The following molecule contains the functional groups 2. A. primary alcohol and primary amine C. secondary alcohol and primary amine 3. A. alkene and secondary alcohol C. ketone and seconary alcohol The following molecule contains the functional groups B. ether and ketone D. ether and alkene 4. The following molecule A. primary amine and ketone C. secondary amine and alkyne NH₂ contains the functional groups B. secondary amine and alkene D. secondary amine and ketone ОН OH B. secondary alcohol and secondary amine D. primary alcohol and secondary amine NH₂ OHarrow_forwardii) The two substances shown above would best be described as: a. different compounds b. structural isomers c. geometric isomers d. aliphatic hydrocarbons e. aromatic hydrocarbonsarrow_forward
- What functional groups are contained in the molecule below? methyl amino phosphate carbonyl carboxyl hydroxylarrow_forwardDraw a STRUCTURAL DIAGRAM FOR the following organic compounds. 1.1-aminopropane 2. butyl ethanamide 3. methanal 4. 3-hexanone 5. ethyl pentanoate 6. ethynearrow_forwardDetermine the functional group(s) for the following molecule (choose all that apply).CHOOSE ALL THAT APPLY(It is NOT hemiacetal and ester, I already tried and it was wrong) a. hemiacetal b. phenol c. acetal d. carboxylic acid e. ester f. aromatic g. ketone h. aldehydearrow_forward
- 3. Functional groups and application to human body for steroids ?arrow_forwardA. Molecules that contain which two functional groups are likely to have the highest boiling point? B. Which functional groups are non-polar?arrow_forwardUse IUPAC rules to draw and/or name the following organic molecules. (you can draw the carbon chains only with functional groups...) A. Pentanol B. Butanal C. Ethanoic acid D. CH3C=OCH2CH3arrow_forward
- Refer to the given structure and answer the following questions:arrow_forwardExplain what is wrong with the following organic molecule names, then give the correct name. a. 3-chlorobutane b. 2-pentanal c. 2,3,4-triethyl-hexane d. cis-2-methyl-2-butenearrow_forwardPlease do all parts of the questionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY