
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
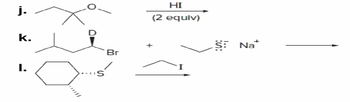
Transcribed Image Text:j.
k.
Br
I.
HI
(2 equiv)
::
S:
Na+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Consider the following balanced reaction: 2VO43- (aq) + SO₂ (g) + 8H+ (aq) --> 2VO₂ (aq) + SO4²- (aq) + 4H₂0 (1) Match the following elements and compounds to their correct description: A: V B: O C: S D: H 3- E: VO4³- F: SO₂ G: H+ A C D B ✓ [ Choose ] is the oxidizing agent is the reducing agent is neither the oxidizing agent nor the reducing agent is reduced is oxidized is neither oxidized nor reduced [Choose ]arrow_forwardMass of crude Acetanilide = 0.508 Mass of filter paper = 0.800g Filter paper + pure Acetanilide = 1.153g Mass of pure Acetanilide = ? % recovery = ? Mpt of recrystallized Acetanilide =112 C – 114 C Mpt of crude Acetanilide = 97 C – 110 Carrow_forwardlogi *(X Con (110 M Inb Doc (10 D (10 Doc M You 88 Nex Luk0 Mic tOP m/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take erosoft Office Ho... Imported From Fire... G3 Google Drive GK3 G1-Google Drve New Guiding Cours... SL What did you do la.. [References] A 2.66-g sample of a pure compound, with formula MSO̟, was dissolved in water and treated with an excess of aqueous calcium chloride, resulting in the precipitation of all the sulfate ions as calcium sulfate. The precipitate was collected, dried, and found to weigh 1.36 g. Determine the atomic mass of M, and identify M. Atomic mass = Element M: Try Another Version 3 item attempts remaining Submit Answerarrow_forward
- The literature value for the heat of solution of KNO3 is 34.89 kļ/mole. Your experimental value is 29.5 kl/mole. What is the accuracy of your determination as a relative percent error? O -7.3% O -15.4% O -18.3% O -84.6%arrow_forward2. 75(10 followinm Reaction: at ded dinap 302 T2 020arrow_forwardLimiting reagent and theoretical yield.arrow_forward
- 2.3arrow_forwardConstants I Periodic Table Part A For the following reaction studied in the gas phase (Fridgen, T. D., et al. J. Phys. Chem. A 2005, 109, 7519): Ci-+ C3H7CN → C1C3H7+ CN- Is the overall reaction exothermic or endothermic? Match the words in the left column to the appropriate blanks in the sentence on the right. the following potential energy surface was derived: [Cl–C;H;-CN]¯ Reset Help ClC;H,+ CN exothermic The overall reaction is because at a higher energy endothermic than the products are the reactants are Cl +C;H¬CN the transition state is [CIC;H,-CN] Submit Request Answer [Cl–C;H;CN] Reaction progress Part B The mechanism for the reaction, based on the potential energy diagram, There are six activation energies, one associated with each of the rate constants in the mechanism. Two of the elementary steps are barrierless, in that they have activation can be written as: energies equalling zero. Which of the six activation energies, Eal, Ea–1 Ea2 Ea-2 Ea3 Ea-3 are equal to zero? k…arrow_forwardF Consider the retrosynthesis of the following target molecule. Provide at least two possible starting materials that could be used to prepare the target molecule. Select the compounds (plus reagents) that would be suitable starting materials for an efficient synthesis of the given target molecule. Select all that apply. 2 W S 0 X 0 JOH # BAI 3 OH + NaOMe x OH e Br + NaOMe d + conc. H₂SO4 and heat C C $ 4 r f % 5 V t 6.0 6 b Oll y h & 7 O n u * 00 8 j m 9 k O < 0 alt рarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY