
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
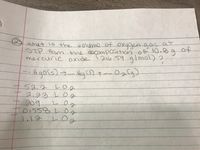
Transcribed Image Text:unat is the wolume of oxygengasa
STP from the docomposition.of 10.@g
9lmol)?
of
mercuricoxide l2i6:59 almo 2
Hgors)7-HaD+--Oalg)
752.2LOg
2.23L0
f 209
T0:558 LOg
1,17L0
தடு.செ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 125. mL Bample Ot ehlonine gas at 25°C is heated to a volume of 175 mb, if the Pressnte remains Constant, uhat is the final Celsius vemperature ?arrow_forwardCHAPTER / UAJLJ, O Previous Page 11 of L6 Next O A balloon containing 7.4 Lair at 28.0°C is placed in a freezer at -19.0°C. What is the new volume of the balloon?arrow_forwardAt STP, 23.50 mol of gas occupies approximately what volume in liters?arrow_forward
- ghment-tak! Google Keep E Economics-Googl. Music d nenos ..: nom Algebra Norten ondial Arkansas D. Video Library America An expandable vessel contains 693 ml of gas at 23 °C. What volume will the gas'sample occupy if it is placed in a bolling water bath (100. °C)? Volume = mlarrow_forward5-What volume of hydrogen gas at STP is produced from the reaction of 31.49 g of Mg? Balance the equation: ____Mg + ____ HCl -> ____MgCl2 + ____H2arrow_forwardMotivation: Answer the question below. How do we explain the behavior of gasses? 2 Pre-Assessment/APK: Complete the table below by giving the equivalent of quantity in terms of other units. Quantity Millimeter Mercury C en mm Hg)nfanimatab al cuee Pascal( Pa) d Pounds per square inch(psi) 14.7 Standard Torr atmosphere(atm) 1 Atm 1.32 x 10-3 1.32 x 10-3 9.87 x 10-6 Sbap 0.068uoenumle 016 enuts1egmtbns ermulov STeig nertw esp s o1 n9s lw JunW Torr 1 133.32 mm Hg 1 133.32 Pa psiarrow_forward
- chemistryarrow_forwardIf there are 1.4 x 103 mg of chlorine gas at STP, how many liters does this occupy? to the nearest thousandthsarrow_forwardASES are of C hip bet Charles tions resul er at unl ar er m V 6 7 8 5 3 2 4 Moles of CO₂ gas produced (use stoichiometry) 1 Pressure of CO₂ gas (atm) Rearrange the Ideal Gas Law equation! Part B: Graphing Example Data to Describe Boyle's Law Complete the final 2 columns in the example data table below. Plot a graph of Volume vs. Pressure. A DAT Plot a second graph of Volume vs. 1/Pressure. I will accept graphs done by hand or in Excel. As before, submitted graphs need to have all the pieces of a complete & correct graph that were first explained in Lab 2. Reminder: Boyle's Law assumes the temperature & amount of gas are both kept constant, which is why those factors are not in the equation. Reading Pressure (mm Hg) 675 690 705 720 735 750 765 780 Volume (mL) 1145 1083 1021 959 897 835 773 711 Use your graphs to answer these questions: be eranc 1/ Pressure PV (product) gripini babbs 03 to 22601 156X SA1 dd (mo) Questions If the pressure of the gas is 760 what volume (in mL) would…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY