
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Limiting reagent and theoretical yield.
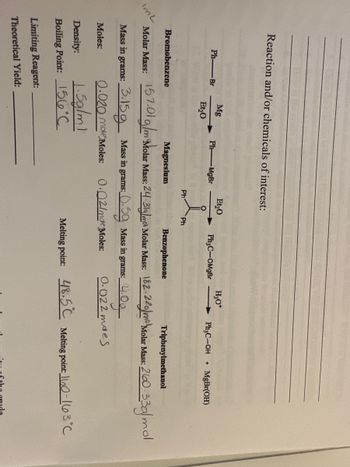
Transcribed Image Text:Reaction and/or chemicals of interest:
ImL
Ph-Br
Bromobenzene
Mg
Et₂0
Moles:
Ph-MgBr
Molar Mass:
Mass in grams: 3.159 Mass in grams:
0.020 mole Moles:
Density:
1.5g/m1
Boiling Point: 156°C
Limiting Reagent:
Theoretical Yield:
Ph
Et₂0
Ph
Ph₂C-OMgBr
Magnesium
Benzophenone
Triphenylmethanol
157.01 g/m Molar Mass: 24:3ly/mro Molar Mass: 182-220) Molar Mass: 2600-33g/mol
H₂O*
5: 0.5g Mass in grams:
0.02/mde Moles:
Ph3C-OH +
MgBr(OH)
4.0g
0.022 maes
Melting point: 48.5 Melting point: 1100-13°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Suppose 1.0g of Compound A and 9.5g of Compound B are consumed in a reaction that produces only one product, Compound C. Calculate the theoretical yield of C. Round your answer to the nearest 0.1g. Suppose 9.6g of C are actually isolated at the end of the reaction. What is the percent yield of Compound C?Round your answer to the nearest whole percent.arrow_forwardTheoretical yield in grams and percent yield (show calculation below) of butryic acid + ethanol= ethyl butyrate. Also, two compounds were removed during the sodium bicarbonate washes, draw the acid/base reactions in question and explain how your product is purified due to these reactions occuring. actual yield= 1.2 grams ethanol= 3 mL butryic acid= 5 mLarrow_forwardYou are performing an extraction and you begin with 0.70 g of crude starting material. At the conclusion of this exercise, you isolate 0.52 g of pure product. Calculate a percent recovery?arrow_forward
- 1) Explain the difference between a limiting and excess reagent. 2) A group of friend create an open-air campfire outside which burns wood. Carbon dioxide and water are produced from the burning.arrow_forwardIf a student records a percentage yield of more than 100%, what are the possible errors? Select all that apply O not washing the product well enough thereby comtaminating the product with other side products formed during the reaction. incomplete reaction that led to loss of product loss of product during a separation process in the experiment O not completely drying the product before weighing the actual yield.arrow_forwardYou would like to buy a monthly compounding 15-year, $1,000 par value bond. The yield to maturity of this bond is 12 percent. If the annual coupon rate of the bond is an 8.5 percent, what is the value of this bond? Is this bond selling at discount or premium? Group of answer choices $757; Discount $1,225; Premium $1,296; Premiumarrow_forward
- The amount of product collected after performing a lab experiment is the A. actual yield B. theoretical yield C. percent yield D. limiting reagentarrow_forwardNeed help asap! Thanks!!arrow_forwardmass of Erlenmeyer flask + 2-methyl-2-butanol 35.39g mass of Erlenmeyer Flask 27.72g mass of 2-methyl-2-butanol 7.67g 40.67G Mass of 2-chloro-2-methyl butane + bí 33.35G Mass of preweighed round bottom flask (rbf 7.32G Mass of 2-chloro-2-methyl butanearrow_forward
- Use the following atomic weights and quantities to calculate the overall % yield of hexaphenylbenzene using the following abstract equation. Assume that all the inorganic reagents are in excess but remember that your yield must be based on the stoichiometry, the limiting reagent, and that grams must be converted to moles. Filling out most of the chart will help. Give only two significant digits in your answer. If after rounding the answer is a whole number, do not include a decimal point. C=12, H = 1, CI = 35.5, O= 16, P = 31 3 Ph Ph- Fe Ph Ph. CI B12 2 Ph H4NO N Phi OH Phi Ph Ph =C: 0 Fe Ph Ph 2 HBr N₂ Ph -Ph C 5 H₂O Ph HC1 H₂ formula formula weight benzaldehyde phenylacetic acid benzyltriphenylphosphonium chloride -> hexaphenylbenzene milliliters 3.843 16.754 density 1.044 grams moles Answer: 5.982 5.592 % yield ?arrow_forwardsandwiches. Assume that sausages only come in packs of five and buns in ingredients, they are the O SEP Use a Model Write a balanced equation for the production of sausage Packs of eight. How many sandwiches can you make if you have one pack of each ingredient? 3 Limiting Reagent and Percentarrow_forwardWhat is the Actual yield and Percentage yield of calcium hydroxide?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY