
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
In this experiment 4 dialysis tubes filled with unknown starch concentrations were placed into beakers with 10% starch solutions. The bags were weighed every 10 minutes for an hour to measure osmosis. Use the figure to answer the following questions.
Which bag(s) was(were) in a hypertonic environment? Select any that apply. Question options:
|
Bag 1 |
|
|
Bag 2 |
|
|
Bag 3 |
|
|
Bag 4 |
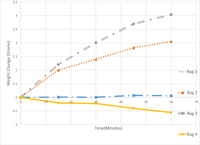
Transcribed Image Text:The graph illustrates the weight change over time for four different bags labeled as Bag 1, Bag 2, Bag 3, and Bag 4. The x-axis represents time in minutes, ranging from 0 to 70, while the y-axis shows the weight change in grams, ranging from -1 to 3.5 grams.
**Bag Data:**
- **Bag 1**: Represented by blue lines with "x" markers, Bag 1 shows a slight increase in weight over time, remaining fairly constant after an initial rise, with changes relatively minimal throughout the 70 minutes.
- **Bag 2**: Indicated by orange dotted lines with asterisk markers, Bag 2 exhibits a slight increase in weight initially, followed by fluctuations around the 0.5-gram mark, with no substantial overall change.
- **Bag 3**: Shown by gray dashed lines with diamond markers, Bag 3 demonstrates a consistent increase in weight, reaching about 3 grams by the end of the 70-minute period.
- **Bag 4**: Displayed with a solid yellow line and circle markers, Bag 4 begins with a slight weight increase but then decreases, reaching approximately -0.5 grams by the 70-minute mark.
This graph visually represents the dynamic changes in weight for each bag over the 70-minute observation period, highlighting different trends such as increase, stability, and decrease in weight among the bags.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- You were introduced to osmosis in Chapter 8 earlier in the course. This week, Chapter 15 continues this discussion by introducing the cell membrane. For additional information, review one or two of the websites listed below. Osmosis: Real-life Applications How Is Osmosis Used in Kidney Dialysis? Guide to Forward Osmosis Applications Choose one way that osmosis and membranes are used in living things, medicine or industry. Describe how osmosis and membranes are used in the real-life application you chose. What are some of the materials necessary? Is an input of energy necessary?arrow_forwardYou set up the following two tubes: Tube 1: 1mL of alkaline phosphatase + 4mL of 400 µM p-NPP Tube 2: 1mL of alkaline phosphatase + 4mL of 200 µM p-NPP Which tube would have a faster reaction rate? Explain your reasoning (why does concentration of p-NPP matter? Relate it back to week 4 lab concepts). Week 4 lab concepts: - concentration gradient - differentially permeable membrane - solute, solvent, diffusion, osmosis, hypotonic, isotonic, hypertonic, molarity, osmolarityarrow_forwardCan you do the first 2 question for me pleasearrow_forward
- Plate A = filter paper dipped into 5% solution of unknown Dye A %3D Plate B = filter paper dipped into 5% solution of unknown Dye B Both plates were incubated at 37°C. At the end of the 60 minute incubation period the diffusion rings for each dye were measured. The diffusion ring for Dye A = 40 mm. The diffusion ring for Dye B = 24 mm %3D Based on the results, which dye had the higher molecular weight? Explain your answer.arrow_forwardYou are working in an emergency care center. A person comes in dehydrated and the physician orders a hypertonic solution. Explain why this solution is used instead of an isotonic solution.arrow_forwardThe cell membrane is permeable to water but not to ions. Select all that apply. Question options: Beaker A is isotonic to the cell. Some water in cell A is moving in and out of the cell. There is a net movement of water into Cell B. Beaker C is hypotonic to Cell C.arrow_forward
- The beaker is divided into two compartments (C and D) that has equal volumes of solution separated by an artificial membrane that has a pore size of 23 Å (Angstrom). Explain the movement of solute after eight hours if comparment C has 17 % sucrose while the compartment D has 2 % sucrose (diameter of sucrose molecule = 9 Å (Angstrom))arrow_forwardOn the graph that relates absorbance to flavin concentration, draw a horizontal line that starts at the point on the y-axis that corresponds to the absorbance of your unknown sample and ends at the curve you drew through your data points . Next draw a vertical line that starts at the point where the horizontal line intersected the curve and ends at the x-axis. The point where this vertical line intersects the x-axis corresponds to the concentration of flavin in your unknown sample. What was the letter on the tube with your unknown sample? What is the concentration of the flavin in your unknownarrow_forwardAn artificial cell consisting of an aqueous solution enclosed in a selectively permeable membrane (but with no cell wall) is immersed in a beaker containing an aqueous solution. The outside environment concentration consists of 0.01 M glucose and the inside of the cell has a concentration of 5.0 M glucose. The plasma membrane is permeable to water and monosaccharides, but impermeable to the disaccharides. Complete the following for the image below: Is the glucose going down or against its concentration gradient? Is the movement of the solute in the cell going out of the cell? Down...Yes Down...No Against...Yes Against...Noarrow_forward
- A 5mL sample diluted 1:1 with trypan blue is counted using a hemocytometer. A total of 325 viable cells and 52 dead cells were counted in the four corner square and the big central square of the meter. What is the total number of dead cells per mL of the sample?arrow_forwardVarious chemical methods can be used to permeabilize or lyse cells including the use of enzymes. List three important factors to consider when developing a lysis method using enzymes. Estimate the osmotic pressure drop across the membrane of an animal cell in a 0.10 M NaCl solution, assuming that the internal total solute concentration is 0.36 osM and temperature is 30.0°C. Do you think that this pressure drop would lyse cells? Why or why not?arrow_forwardA 139 lb male patient with severe lactate acidosis is given isotonic sodium lactate. The doctor orders isotonic sodium lactate 50. mL/ kg body mass to be administered intravenously. The rate of flow is 150 gtts/min, and the IV administration set delivers 20. gtts/mL, where the unit “gtts” stands for drops of liquid. Calculate what is the running time in minutes will be? Show all your calculationsarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education