
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
You set up the following two tubes:
Tube 1: 1mL of alkaline phosphatase + 4mL of 400 µM p-NPP
Tube 2: 1mL of alkaline phosphatase + 4mL of 200 µM p-NPP
Which tube would have a faster reaction rate? Explain your reasoning (why does concentration of p-NPP matter? Relate it back to week 4 lab concepts).
Week 4 lab concepts:
- concentration gradient
- differentially permeable membrane
- solute, solvent, diffusion, osmosis, hypotonic, isotonic, hypertonic, molarity, osmolarity
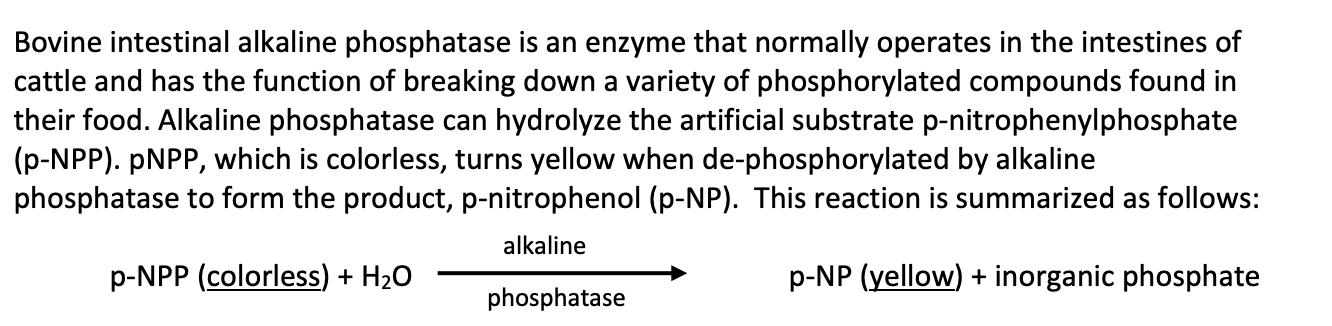
Transcribed Image Text:Bovine intestinal alkaline phosphatase is an enzyme that normally operates in the intestines of
cattle and has the function of breaking down a variety of phosphorylated compounds found in
their food. Alkaline phosphatase can hydrolyze the artificial substrate p-nitrophenylphosphate
(p-NPP). PNPP, which is colorless, turns yellow when de-phosphorylated by alkaline
phosphatase to form the product, p-nitrophenol (p-NP). This reaction is summarized as follows:
alkaline
p-NPP (colorless) + H20
p-NP (yellow) + inorganic phosphate
phosphatase
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Acid phosphatases are an important group of enzymes that can be detected in human blood serum. Under slightly acidic conditions (pH 5.0), this group of enzymes can hydrolyze biological phosphate esters as follows in the picture Acid phosphatases are produced and can be detected in erythrocytes, kidney, spleen, the liver, and prostate gland. The enzyme from the prostate gland is clinically important because an increased activity in the blood is frequently an indication of cancer of the prostate gland.Tartrate ion can strongly inhibit the phosphatase from the prostate gland, but not acid phosphatases from other tissues. How can you use the information above to develop a specific procedure for measuring the activity of the acid phosphatase of the prostate gland in human blood serum?arrow_forwardIn bacteria, isocitrate dehydrogenase is regulated by phosphorylation of a specific Ser residue in the enzyme active site. X-ray structures of the phosphorylated and the nonphosphorylated enzyme show no significant conformational differences. How does phosphorylation regulate isocitrate dehydrogenase activity? O The phosphoryl group sterically hinders the substrate. O The phosphorylation bears a negative charge, which repels the substrate. O The phosphoryl group attracts positively charged Ca2* cations, which block the active site on the enzyme. None of the above.arrow_forwardIn a cell with abundant energy (in the form of ATP and NADH), and also glucose and other carbohydrates to break down, it might be advantageous to store some of that glucose in the form of glycogen. Which of the enzyme(s) (hexokinase, G6P isomerase or PFK-1) do you think is most likely to be inhibited in order to divert those carbohydrates into the synthesis of glycogen for storage? Why?arrow_forward
- The effect of ATP on the allosteric enzyme PFK-1 is shown below. For a given concentration of fructose 6-phosphate, the PFK-1 activity increases with increasing concentrations of ATP, but a point is reached beyond which increasing the concentration of ATP inhibits the enzyme. (a) Explain how ATP can be both a substrate and an inhibitor of PFK-1. How is the enzyme regulated by ATP? (b) In what ways is glycolysis regulated by ATP levels? (c) The inhibition of PFK-1 by ATP is diminished when the ADP concentration is high, as shown in the illustration. How can this observation be explained? *A graph is included for this question*arrow_forwardWhy does the lack of glucose 6-phosphatase activity in the brain and muscle make good physiological sense? Glucose 6-phosphatase allows cells to trap glucose in the cell; however, these tissues primarily rely on noncarbohydrate energy sources. Glucose 6-phosphatase allows cells to generate glucose through gluconeogenesis; however, gluconeogenesis only takes place in the liver. Glucose 6-phosphatase allows cells to release glucose into the blood; however, these tissues should never release glucose. Glucose 6-phosphatase provides glucose 6-phosphate for glycogen synthesis; however, these tissues do not need glycogen.arrow_forwardesent ane to produce the sucrose is actually points) Consider a situation where valine was used by the liver for gluconeogenesis. Valine is metabolized via the following pathway. 2022, Arizona State University COA valine NH₂ COO CO₂ ATP HỌ NHỉ COA CO₂ COO NAD NADH NAD NADH COA H+ H FAD FADH2 r CO2 COA COA H₂O COO H COO NADH NAD+ H HOT GTP GDP HOT P₁ COA ADP+ P COA COO COA Coo succinyl CoA How many molecules of valine would be required for one molecule of glucose? Using the appropriate amount of valine determined above, how many NADH, FADH2, and ATP would be produced or consumed by the conversion of valine into glucose? Assume that any net production of NADH and FADH2 is used in electron transport. What is the maximum net ATP production in the human mitochondria (10 c subunits)? Don't forget to add/subtract the ATP determined in the above question.arrow_forward
- The Beutler test is used to diagnose GALT (UDP-glucose uridyltransferase) deficiency in infants. Blood from the heel is spotted onto filter paper and the spot is then subjected to an enzyme assay. Galactose 1-phosphate, NADP+ and UDP-glucose are added as substrates to the dried blood. The increase in absorbance at 340nm is measured over time which corresponds to reduction of NADP+ to NADPH . The amount of GALT activity in the blood of patients is therefore measured by stoichiometric relationship to the amount of NADPH produced from NADP+. However, GALT does not directly reduce NADP+ as you can see in the above diagram. Instead, the glucose 1-phosphate product from this reaction is shuttled into the oxidative phase of the pentose phosphate pathway by enzymes also present in RBCs. Which three (or possibly more if you must) enzymes downstream of GALT allow for quantitative correlation of a product of GALT with the appearance of NADPH? What is the stoichiometric relationship…arrow_forwardConsider the mechanism of enolase, as indicated below. Which of the following correctly describes the roles of the Mg2+ as illustrated in the figure? (This is a multi- select question). Mg2+ Mg2 Enolase PO3- OH -C-C-H H OH HO H-N-H Lys 345 Glu211 2-Phosphoglycerate bound to enzyme Mg2+ Mg2 PO3- OH C-C-H OH HO H H-N*-H Lys 345 O Glu211 Enolic intermediate HOH PO3- H Phosphoenolpyruvate The metal ion (Mg2+) is helping to stabilize the extra negative charge that developed on the carboxyl group in the enolic intermediate. The metal ion (Mg2+) is serving as a general base, removing a proton in order to improve the quality of the nucleophile. The metal ion (Mg2+) is assisting in the oxidation of the carboxyl carbon through metal ion catalysis. The metal ion (Mg2+) is helping to orient the substrate properly in the active site. The metal ion (Mg2+) is accepting a proton in order to improve the quality of the leaving group.arrow_forwardStrategies for regulating the central pathways in carbohydrate metabolism vary among different cells in one organism and among organisms. Slight changes in the regulation of enzymes in central metabolism can effectively re-route metabolite traffic through these pathways, just like a small mutation in PFK-1 can convert a healthy cell into a cancerous one. For instance, Gillaspera mold uses an alternative strategy for regulation the TCA and glycolysis. Gillaspera contains a unique isocitrate dehydrogenase that has an allosteric site for citrate. High citrate inhibits isocitrate dehydrogenase in this organism. Gillaspera also lacks a citrate binding site on PFK-1, so this variant of the enzyme is not affected by citrate concentrations at all. Gillaspera lacks the enzymes ethanol dehydrogenase and lactate dehydrogenase and no carbons are lost in its unique fermentation product. Would high glucose in these organisms lead to production of carbon dioxide from glucose catabolism?…arrow_forward
- Glucose is phosphorylated to glucose-6-phosphate by hexokinase in the first step of the glycolytic pathway to trap it in the cell, as G6P cannot diffuse across the lipid bilayer. This reaction also decreases the concentration of free glucose, favoring additional import of the molecule. However, this has a postive standard free energy of 3 kcal per mole. To favor this reaction, hydrolysis of ATP is coupled, which has a standard free energy of -7 kcal per mole. Determine the actual free energy change in kcal/mole for the following conditions: Glucose concentration: 4.5 mM ATP conc: 3.79 mM G6P conc. 0.052 mM ADP conc: 0.12 mM inorganic phosphate conc: 1.3 mM temperature: 313 K Ans. in 3 SFs.arrow_forwardIf glucokinase has a higher Km for glucose than does hexokinase, but can only bind to D-glucose, while hexokinase can bind to several hexose sugars (like D-glucose, D-mannose, and D-fructose), then: glucokinase has both a higher affinity and a higher specificity for D-glucose than does hexokinase glucokinase has both a lower affinity and a lower specificity for D-glucose than does hexokinase glucokinase has a higher affinity but a lower specificity for D-glucose than does hexokinase glucokinase has a lower affinity but a higher specificity for D-glucose than does hexokinase all of the abovearrow_forwardThere is another class of aldolase enzymes known as Class II. These enzymes are found in fungi, algae, and some bacteria. This class differs for Class I in that these enzymes do not have a Lys residue associated with their active sites, but contain a divalent cation (usually Zn2+ or Fe2+) in the active site. Outline a possible mechanism for a Class II aldolase and explain the function of the metal ion in the reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education