
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
In the separation process illustrated below, fresh feed stream F consists of 20% A and 80% B on a mass basis. F mixes with recycle stream R to produce mixed stream M, which enters the separator. The mass ratio of A:B in stream M is 2:3. Product stream P, which leaves the separator at 100 kg/h, is pure A, while waste stream W is 5 mass % A. Recycle stream R is 80 mass % A.
(a) Find all unknown stream flow rates.
(b) Calculate the recycle ratio.
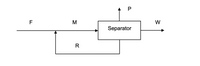
Transcribed Image Text:P
F
M
W
Separator
R
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 6. During Basic Oxygen Process to manufacture low carbon stainless steel, 600 kg of cast iron (6% of carbon), 500 kg of cast iron (5% of carbon) and 400 kg of cast iron (4% of carbon) will be mixed with household scrap of steel (2% of carbon) to generate 1800 kg of final product of steel with 0.5% of carbon. a. Write down material balance for iron in feed and final product. Then calculate x. b. Write down material balance for carbon in feed and final product. Then calculate total carbon removed (kg) with the slag. Basic-onygen-fornace steelmaking (Lina and Donawita prnceu) Feed: • 600 kg cast iron (6% carbon) • 500 kg cast iron (5% carbon) • 400 kg cast iron (4% carbon) • 100 X kg household scrap (2% carbon) Molten iron (70-75%6) + steel scraps (25-30)+ limeidolomite Refractory lining. Osygen lance Tap hole Pure O carbon Slag Steel BOF slag BOF converter Product: 1800 kg stainless steel (0.5% carbon)arrow_forwardL-serine is produced by fermentation and recovered and purified by crystallization at 10oC. To increase the yield of the crystallization step, methanol is added to the system. An aqueous solution containing 27wt% L-serine is added with methanol to a batch crystallizer and cooled to 10oC. The crystals are then recovered by filtration. Draw a flowchart for this process showing the key unit operations with appropriate stream labels and composition.arrow_forwardCalculate the % yield for each crude solid based on the total amount of starting mixture. Starting mass = .804g Mass of crude p-dimethoxybenzene = .401g Mass of benzoic acid = .396garrow_forward
- 15.00 ml of a 0.3400 m manganese(iii) acetate solution is mixed with 25.00 ml of a 0.2311 m ammonium carbonate solution. Calculate the number of grams of solid precipitate produced. Calculate the molarity of all species in solution after the reaction has taken place.arrow_forward1,3,5 trimethylbenzene may be reacted with hydrogen over a catalyst to form meta- xylene, which is a valuable product at $1.35/kg. However, meta-xylene may also react with hydrogen to form toluene, which is much less valuable at $0.50/kg. Methane is a side product of both reactions, and it gets recycled elsewhere in the plant. 1 kg of catalyst is degraded for each 200 kg of toluene produced, and it must be disposed of in a toxic waste landfill at a cost of $25/kg. It is proposed to alter the conditions of the reactor such that the selectivity of the reaction is increased from 0.8 (the current selectivity) to 0.9 (the proposed selectivity). Calculate the amount of money gained (in dollars) by making this change to the process, for each 100 kg of 1,3,5 trimethylbenzene reacted.arrow_forwardThe zeolite LTA is used as an adsorbent for separations. A nitrogen adsorption study of LTA was performed (0 °C, PN₂= 760 mmHg). Using the BET plot below, calculate the specific surface area (in units of m²/g) of the zeolite. The density and molecular weight of N₂ are 0.808 g/cm³ and 28 g/mol, respectively. Assume nitrogen is a sphere with the projected surface area a (cm²/molecule). Recall that the molar volume of an ideal gas at STP is 22,400 cm³/mol.arrow_forward
- Reverse Osmosis is used by water bottling companies to produce desalinized water from sea water. Giventhe figure below, determine the fraction of brine leaving the RO cell that is recycled.arrow_forwardAniline is produced by the hydrogenation of nitrobenzene. A small amount of cyclohexylamine is produced as a by-product. Nitrobenzene is fed to the reactor as a vapor with three times the required stoichiometric amount of hydrogen. The conversion of nitrobenzene to the products is 96% and the selectivity to aniline is 85%. Unreacted hydrogen is separated from the reaction products and recycled to the reactor. From the recycle line it is purged to keep the inerts in the recycle stream below 5%. The fresh hydrogen that is fed is 99.5% pure and the rest is inert. Calculate the adiabatic outlet temperature of the reaction products and indicate the relationship with respect to the reference temperature (298.15) (in K):arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The