
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
The zeolite LTA is used as an adsorbent for separations. A nitrogen adsorption study of LTA was performed (0 °C, PN₂= 760 mmHg). Using the BET plot below, calculate the specific surface area (in units of m²/g) of the zeolite. The density and molecular weight of N₂ are 0.808 g/cm³ and 28 g/mol, respectively. Assume nitrogen is a sphere with the projected surface area a (cm²/molecule). Recall that the molar volume of an ideal gas at STP is 22,400 cm³/mol.
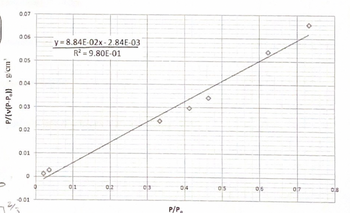
Transcribed Image Text:P/[v(P-P.)}. g/cm³
0.07
0.06
0.05
0.04
0.03
0.02
0.01
0
-0.01
213
0
Po
y=8.84E-02x-2.84E-03
R² = 9.80E-01
01
012
03
D
04
P/P
O
05
06
017
018
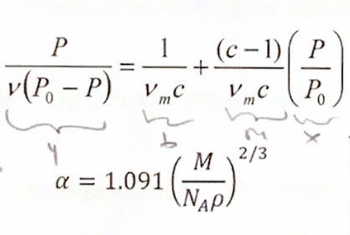
Transcribed Image Text:P
1
v(P-P) VmC
4
α = 1.091
+
(c-1)
VmC
M 2/3
(NAP)
P
Po
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Similar questions
- nitrogen. 25.20 Determine the total nitrogen concentration in mg/L of a water sample containing 1.3 mg/L ammonia, 0.25 mg/L nitrite, 15.1 mg/L nitrate, and 0.9 mg/L organic nitrogen.arrow_forward1.6 Properties: Diffusivity Estimate the molecular diameter and diffusion coefficient for the proteins ribonuclease (MW 13,700 Da), hemoglobin (MW 68,000), and urease (MW 480,000), assuming the molecules are spherical and the density of each protein molecule is 1.3 g/cm³.arrow_forwardAn Al-4.5wt%Cu alloy has a low-temperature yield stress of 600 MPa due to the precipitation strengthening effect imparted by the equilibrium q phase (Al2Cu) precipitates. Estimate the interparticle spacing and particle size in this alloy. Given: density of α-solid solution 2,700 kg/m3 and density of q phase 4,430 kg/m3. Assume the Cu content in α-solid solution 0.5 wt% and the Cu content in the q phase 54%. Hint: You need to use the Orowan model of particle strengthening and the lever rule among other concepts.arrow_forward
- Bakelite is a polymer widely used in the manufacture of housings for electronic devices, its monomeric form is presented below: Knowing that you have an average molecular weight of the polymer of 700000 g / mol, determine the degree of polymerization .arrow_forwardThe percent by mass of phenol (MM= 94.11 g/mol) in an aqueous solution is 10.9%. What is the molality of the phenol solution?arrow_forwardBased on the graph below, if 70.0 g of HCI are dissolved in 100 g of water at 40 degrees Celsius is the solution saturated, unsaturated, or supersaturated? Table G Solubility Curves NANO, KI 140 130 KNO, 120 110 100 90 80 NH,CI 70 HCI 60 KCI 50 40 NaCI KCIO, 30 20 NH, 10 10 20 30 40 50 60 70 80 90 100 Temperature (°C) Solute per 100 g of H,0 (g)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The