
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
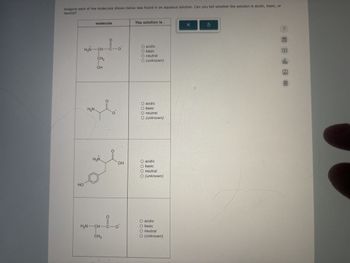
Transcribed Image Text:Imagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or
neutral?
molecule
0=0
H3N-CH-C-o
HO
CH2
OH
The solution is...
O acidic
O basic
O neutral
O (unknown)
H₂N
acidic
O basic
O neutral
○ (unknown)
+
H3N
O
OH
O acidic
O basic
O neutral
O (unknown)
H2N-CH-C-O
CH3
O acidic
O basic
neutral
○ (unknown)
X
?
olo
HE
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- You are asked to calculate the H+ concentration in a solution of NaOH(aq). Because sodium hydroxide is a base, can we say there is no H+. since having H+ would imply that the solution is acidic?arrow_forward1. Which of the following can act as a Lewis acid? (Hint : In each case, draw the Lewis electron dot structure of the molecule or ion. Are there lone pairs of electrons on the central atom? If so, it can be a Lewis base. Does the central atom lack an electron pair? If so, it can behave as a Lewis acid.) PH3 BCl3 H2S HS−arrow_forwardWrite equations to illustrate the acid-base reaction when each of the following pairs of Brnsted acids and bases are combined: Acid Base a.HOCl H2O b.HClO4 NH3 c.H2O NH2 d.H2O OCl e.HC2O4 H2Oarrow_forward
- Use Table 13-2 to order the following from the strongest to the weakest base. ClO2,H2O,NH3,ClO4arrow_forwardUse Table 14.3 to help order the following acids from strongest to weakest HNO3,H2O,NH4+,C5H5NH+arrow_forwardRank the compounds in each of the following groups in order of increasing acidity or basicity, as indicated, and explain the order you assign. (a) acidity: HCI, HBr, HI. (b)basicity: H2O, OH-, H-, CI-. (c) basicity: Mg(OH)2, Si(OH)4, ClO3(OH) (Hint: Formula could also be written as HCIO4). (d) acidity: HF, H2O, NH3, CH4arrow_forward
- Novocaine, C13H21O2N2Cl, is the salt of the base procaine and hydrochloric acid. The ionization constant for procaine is 7106. 15 a solution of novocaine acidic or basic? What are [H3O+], [OH-], and pH of a 2.0% solution by mass of novocaine, assuming that the density of the solution is 1.0 g/mL.arrow_forwardCalculate H+ and OH- and in solutions with the following pH. (a) 9.0(b) 3.20 (c) -1.05 (d) 7.46arrow_forward. Consider 0.25 M solutions of the following salts: NaCl. RbOC1, KI, Ba(ClO4),, and NH4NO3. For each salt, indicate whether the solution is acidic, basic, or neutral.arrow_forward
- Will the following oxides give acidic, basic, or neutral solutions when dissolved in water? Write reactions to justify your answers. a. CaO b. SO2 c. Cl2Oarrow_forwardBoth HF and HCN ionize in water to a limited extent. Which of the conjugate bases. F“ or CN”, is the stronger base? See Table 14.2.arrow_forwardConsider two weak acids, HA (MM=138g/mol)and HB (MM=72.0g/mol). A solution consisting of 11.0 g of HA in 745 mL has the same pH as a solution made up of 5.00 g of HB in 525 mL. Which of the two acids is stronger? Justify your answer by an appropriate calculation.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning