
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Assign all the carbons
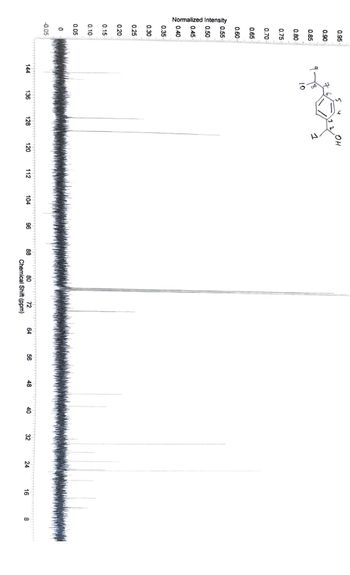
Transcribed Image Text:0.95
0.90
0.85
Malalahluluate
OH
32
マ
10
0.80
0.75
0.70
0.65
0.60-
0.55
0.50
Normalized Intensity
0.45
0.40-
0.35
0.30
0.25
0.20
0.15
0.10
0.05
0
-0.05
144
136
128
120
112
104
96
96
88
88
80
72
64
Chemical Shift (ppm)
56
56
48
40
32
32
24
24
16
16
8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- A. Draw structure of compound. B. Label base peak and molecular ion peak.arrow_forwardFollowing are infrared spectra of 2-methyl-1-butanol and tert-butyl methyl ether. Assign each compound its correct spectrum. Micrometers 2.5 100 3. 4. 9. 13 8 10 11 12 14 15 20 90 80 70 50 40 30 20 10 4000 3600 3200 2800 2400 2000 1800 1600 1400 1200 1000 800 600 400 Wavenumber (cm-1) Transmittance (%)arrow_forwardPercent Transmittance 0.4 0.35 0.3 0.25 0.2 0.15 0.1 0.05 0 4000 N Liquid Sample 1 3500 3000 2500 2000 1500 1000 500 Wavenumber (cm³)arrow_forward
- Is the product pure? how do you know? here is the results: Starting mass of benzyl 1.0883g Collected mass of product 0.8957g Product melting point 135˚C Mixture melting point (product mixed with benzoin) 114˚C Mixture melting point (product mixed with meso-hydrobenzoin) 135˚Carrow_forwardC2H3ClO2 is the formula, the solvent is CDCl3. Need help determining Integration values the multiplicity and the chemical the Peak refers to.arrow_forwardPlease what molecule is thisarrow_forward
- Can you help me label this irarrow_forwardMALI 250 200 150- 100 50 WWWD1A, Wavelength 254 nm (Vuyo2018-09-17Mxture 70% methano 1.5mmin.D) 0.152 2 2016 799 Figure 4 Standard mixture comprising of man wwwwwwwwww toluene, ethylbenzene and cumene 1μL injected at selected isocratic mixture at a flow rate of 1.50 mL/min From Figure 4 decide if the increase in flow rate is beneficial for the analysis time.arrow_forward%Transmittance 2803.84 2530,61 2179.10 1667.25 1610.3 575.42 1514.36 1416.49 1320.29 1281.24 1181.12 1116.52 1039.48021.53" 959.2843.73 835.80 751.45 95 fo radicat 90- 85- 80- 75- 70- 65- 60- 55 50- 45- 40 3500 3000 2500 2000 1500 1000 Wavenumbers (cm-1) Wed Oct 05 06:10:12 2011 (GMT-04:00) FIND PEAKS: Spectrum: Region: Absolute threshold: 90.824 jo radical 3529.29 721.12 Sensitivity: Peak list: 50 751.45 51.334 Position: Position: Position: Position: Position: Position: Intensity: Intensity: Intensity: Intensity: Intensity: Intensity: Intensity: Intensity: 68.728 66.910 835.80 943.73 959.28 1021.53 67.665 78.586 Position: Position: 1039.48 1116.52 79.710 68.993 67.903 1181.12arrow_forward
- 1. Each of the following IR spectra is associated with one of the aromatic compounds below. Identify the compound associated with each spectrum. N. H HO a-tetralone B-tetralone 2-indanone N-methylbenzamide m-cresol Spectrum A MICRONS NICOLET 20SX FT-IR 2.8 2.9 2.5 100 2.6 2.7 3.5 4 4.5 5.5 7 10 11 12 13 14 15 16 17 18 19 21 22 0.0 90 .05 80 0.1 70 % 0.2 60 50 0.3 40 0.4 0.5 30 -0.6 20 0.7 0.8 0.9 1.0 2.0 4000 3800 3600 3400 3200 3000 2800 2600 2400 2200 2000 1800 1600 1400 1200 1000 800 600 450 WAVENUMBERS Spectrum B MICRONS NICOLET 20SX FT-IR 2.5 100 2.6 2.7 2.8 2.9 3 3.5 4 4.5 5.5 7 8 10 11 12 13 14 15 16 17 18 19 21 22 + 0.0 90 .05 80 0.1 70 % 0.2 60 50 -0.3 B 40 0.4 E 0.5 30 0.6 20 0.7 0.8 0.9 10 1.0 2.0 4000 3800 3600 3400 3200 3000 2800 2600 2400 2200 2000 1800 1600 1400 1200 1000 800 600 450 WAVENUMBERS This work by Dr. James S. Nowick, Professor of Chemistry, University of California, Irvine, is licensed under a Creative Commons Attribution 4.0 International License. Spectra…arrow_forwardPlease what molecule is thisarrow_forward(B) Relative intensity 100 15 m/z 20 3.0 40 55 60 71 80 8.6 (CH3)2CHCH₂CH₂NHCH₂CH₂CH(CH3)2 100 100a 100 114 128 120 142 CH₂CH(CH₂ 140 157M+. 160arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning