
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
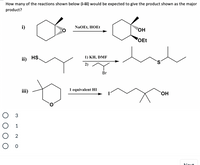
Transcribed Image Text:**Question:**
How many of the reactions shown below (i-iii) would be expected to give the product shown as the major product?
**Reaction Details:**
**i)** Cyclohexanone with an ethyl group is reacted with sodium ethoxide (NaOEt) and ethanol (HOEt) to form a compound with adjacent hydroxyl (OH) and ethoxy (OEt) groups on a cyclohexane ring.
**ii)** A thiol group (HS) attached to a butyl chain reacts with 1) potassium hydride (KH) in dimethylformamide (DMF), followed by 2) 1-bromo-2-methylpropane, resulting in a sulfide with an extended alkyl chain.
**iii)** A cyclic ether reacts with one equivalent of hydroiodic acid (HI) to produce a primary iodide and an alcohol in a six-carbon chain.
**Options:**
- ○ 3
- ○ 1
- ○ 2
- ○ 0
The question asks to determine how many of these reactions correctly result in the depicted major product.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you show the mechanism and show all the stepsarrow_forward6. Mechanisms: Propose a mechanism and major product for the following reactions. OH 1. NaNH2 2. OMs rt OH CIarrow_forwardLearning Activities 7. Show the products that will form in the reactions shown below and summarise the steps involved in words C4H9- NH₂ O NH₂ EtO₂C. -CO₂Et CH3 CO₂Et HON CH3 Ph Pharrow_forward
- The synthesis that is outlined has a flaw in each step. Provide alternate reagents for each step that would yield the product that is shown as the major product.arrow_forwardbiackboard.gwu.edu/webapPps/assessment/feview/review.jsprattempld Select the major product of the reaction below. 1) TIPSCI, Et3N 2) Na 3) 03, then CH3SCH3 4) CH3CH2LI, then H30* 5) Bu4N* F', H2O CI Selected Answer: Он HO Answers: он он он OH OH OH он Select the major product of the reaction below. The answer is there, but please explain step by step.arrow_forwardComplete the reactions given below. (Note that while some questions ask for a final product, some questions lack the starting product)arrow_forward
- Predict the main product(s) for each of the following reactions belowarrow_forwardPlease answer.arrow_forwardGive all the ten products in the following web of reactions. 10) 3) LAH SO, H2SO. 1) 2) HNO3, H2SOA 1) Mg 2) CHCHO 4) ? CH3CH2CI 5) AIC13 START HERE Br2, hv AICI3 6) 7) Zn/Hg, HCI 8) KMNO4 9)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY