
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
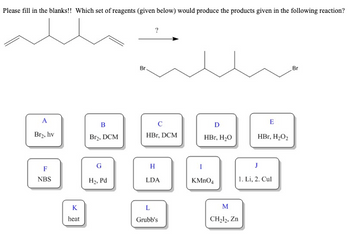
Transcribed Image Text:Please fill in the blanks!! Which set of reagents (given below) would produce the products given in the following reaction?
A
Br₂, hv
F
NBS
K
heat
B
Br₂, DCM
G
H₂, Pd
Br
?
с
HBr, DCM
H
LDA
L
Grubb's
D
HBr, H₂O
I
KMnO4
M
CH₂l₂, Zn
E
HBr, H₂O₂
1. Li, 2. Cul
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Two students are studying the Arrhenius reaction below: NAOH + Hz S04 acid → Na SOy + HzO base salt water Did the students identify the acid, base and salt correctly? If not, provide the correction.arrow_forwardB. Please don't provide the handwriting solutionarrow_forwardfari File Edit View History Bookmarks Window Help DI OD D ¡l A C =5 Previous Page 9 of 9 Next Ⓒ 2 OCT 12 W S S Stv CHAPTER 4-STOICHIOMETRY: QUANTITATIVE INFORMATION ABOUT CHEMICAL REACTION #3 S % by mass E A 10.2 g sample of an aqueous solution of perchloric acid contains an unknown amount of the acid. If 20.5 mL of 1.22 M sodium hydroxide are required to neutralize the perchloric acid, what is the percent by mass of perchloric acid in the mixture? D all A 54 U S R Document3.docx F C ● % 5 C References Use the References to access important values if needed for this question. east.cengagenow.com T с ● FOCUPSia Pxerenc I.Omni Wenarchive G MacBook Pro C Online t... Y 7 H Oct 10. 2022 at 6:04 AM THAN AUD 25 711/7 AL 111.54 AMM U * 3 8 C 9 K 10 KB 747 KR Micros...(.do WAN archivearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY