
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
The synthesis that is outlined has a flaw in each step. Provide alternate reagents for each step that would yield the product that is shown as the major product.
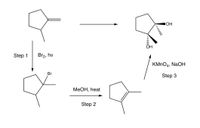
Transcribed Image Text:IOH
OH
Step 1
Br2, hv
KMNO4, NaOH
Br
Step 3
МеОН, һеat
Step 2
.....||
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the following multistep synthesis using appropriate reagents. Indicate the major product after each step and the reaction conditions clearly. steps!!!arrow_forwardProvide a multi-step synthesis of the "Final Product" from the given "Reactant" by completing the synthesis scheme below. Draw the entire synthesis scheme & complete the synthesis scheme and box your answers as shown below. Indicate the set of reagents/conditions #1, #2, and Draw the chemical structure of major organic product at each step i.e. Compounds A, B, and C. Each set of reagents/conditions may contain more than one reagent. Use the notation 1., 2., etc., to show the steps in each set of reagents/conditions as appropriate. Do not show any curved-arrow pushing mechanisms. (Reactant) Final Product TSOH HO OH Compound A Reagents/Condition(s) #1 Compound B MOTHE Reagent(s)/Condition(s) #2 Compound Carrow_forwardCorrect. The reagent is hydroxide, which is both a strong base and a strong nucleophile. The substrate is secondary so we expect both E2 and SN2 processes, although E2 will be responsible for the major product. Accordingly, the major product is the more substituted alkene, with the trans configuration (because the reaction is stereoselective, favoring the trans isomer over the cis isomer). The minor products include the cis isomer, as well as the less-substituted alkene and the SN2 product. Identify the major and minor product(s) that are expected for the following reaction. Select all that apply. PRACTICE the skill Hint Br Choose the major product: 20 10900 botas Practice th OH NaOH OH ? Attempts: 3 of 3 usedarrow_forward
- Help me with chemarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps.arrow_forwardPropose a mechanism for the Pictet-Spengler reaction, an example of which is shown here. Note that a key intermediate is provided. NH2 NH NH H.arrow_forward
- Your goal is to find a way to synthesize the alkyne shown. It may take any number of steps. You must use styrene, but you can use any other reagents you would like to generate the product. ?arrow_forwardSolve it asaparrow_forward3. When the following deuterium-labeled compound is treated with potassium tert-butoxide in DMF, a single product is observed. When the same substrate is heated in the presence of dilute potassium ethoxide in ethanol, a mixture of two products is formed. Provide the complete, detailed mechanism (curved arrows) for each reaction and label each reaction as E1 or E2. Note: deuterium is an isotope of hydrogen and can be treated similarly to hydrogen in chemical reactions but cannot be implied. H KOEt DMF ? D H dilute KOEt EtOH ?+ ?arrow_forward
- Help! Explain in detail please. Thank you! The following alcohol can react with aqeuous sulfuric acid via an E1 reaction mechanism. Draw the mechanism and give the major product of this reaction. Pay attention to stereochemistry if applicable.arrow_forwardPlease help with these and include the steps in the reaction so i can better understand how they work! just 1 or two of them is finearrow_forward10. Draw the complete, detailed mechanism (curved arrows) for the following reaction, and predict the major product. CH3 ▪OH 1. DMSO (COCI)2 2. Et3N Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY