
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Complete the reactions given below. (Note that while some questions ask for a final product, some questions lack the starting product)
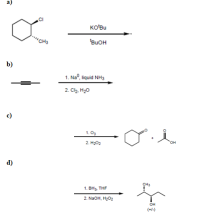
Transcribed Image Text:a)
кови
***CHS
'BUOH
b)
1. Na°, liquid NH3
2. C2, H20
c)
1. O3
2. H202
HO.
d)
1. ВНз THF
2. NaOH, H2O2
он
(+-)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. CH3NH2, TSOH Qarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. || OH (CH3)3CSi(CH3)2Cl, Et3N Drawingarrow_forwardWhat is a major product of the sequence of reactions shown in the box? A) Br 이 ? Pd-cat, Base heat B) D)arrow_forward
- Complete the following synthesis by filling in the missing reactants, reagents, and products.• Boxes with sharp corners are structures; rounded corners are reagents (may be written or drawn).• Reagent boxes may hold multiple reagents/steps.arrow_forward8) Show how the following conversions might be accomplished, including all reagents and intermediates. Br Y. OHarrow_forward3) Draw the major organic product generated in the reaction below. CH3 HCI |arrow_forward
- Provide the product(s) for the following reaction. If no reaction, draw the starting materials.arrow_forwardDiscussion: I want to explain what happened in this reactionarrow_forwardThe addition of an alcohol to an acid chloride is an example of alcoholysis (alcohol addition with bond breakage). Consider the alcoholysis reaction below and answer the questions that follow. 1. Show the tetrahedral intermediate that is formed after the nucleophilic addition of the alcohol to the acid chloride. Be sure to include all lone pair electrons and formal charges on your intermediate structure. 2. Show the final product of this alcoholysis reaction that forms after the intermediate you made in Part 1. Do not include inorganic or charged products in your answer. Be sure to include all lone pair electrons and formal charges.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY