
World of Chemistry, 3rd edition
3rd Edition
ISBN: 9781133109655
Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher: Brooks / Cole / Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
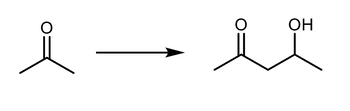
what is the synthesis that would from starting material to product?
Transcribed Image Text:HO
- iN
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 'N Brz NaOH N- ●arrow_forwardAg+KNO3arrow_forward1-86 The specific heats of some elements at 25oC are as follows: aluminum = 0.215 cal/g · oC; carbon (graphite) = 0.170 caI/g oC; iron = 0.107 cal/g mercury = 0.033 1 caI/g oC. (a) Which element would require the smallest amount of heat to raise the temperature of 100 g of the element by 10oC? (b) If the same amount of heat needed to raise the temperature of 1 g of aluminum by 25oC were applied to 1 g of mercury, by how many degrees would its temperature be raised? (c) If a certain amount of heat is used to raise the temperature of 1.6 g of iron by 10oC, the temperature of 1 g of which element would also be raised by 10oC, using the same amount of heat?arrow_forward
- CH,N, Et,0, CH,Cl, 30 min HO,arrow_forwardCH₂-COOH Citric acid has the formula HO-C-COOH I CH₂-COOH CH2–CDD - Na* + HD-C-CDD- Na CH₂-COO- COO-Na A 25.0 mL sample of a concentrated citrus fruit cordial component (e.g. for lime juice), used in the food & drinks industry, was diluted to 250 mL in a graduated volumetric flask. A 25.0 mL sample of this diluted solution, required, on average, 22.5 mL of a standard 0.100 molar sodium hydroxide solution using phenolphthalein indicator for the titration end-point. A) 0.0075 Assuming all the acid in the cordial was citric acid, calculate the concentration of the acid in g/mL in the original solution. B) 0.576 D and is tribasic acid, forming the tri-sodium salt on complete neutralisation with sodium hydroxide. 1.44 0.00225arrow_forward+ 1.03 2. Me₂S od c B A LOHarrow_forward
- K An oil company needs to fill orders for 91-octane gas, but only has 88- and 92-octane gas on hand. The octane rating is the percentage of isooctane in the standard fuel. How many liters of 88-octane gas should be mixed with 300 liters of 92-octane gasoline to get a mixture that is 91-octane gas? Let x be the amount of 88-octane gas used in the mixture. Complete the following chart. Type of Gas 88-octane 92-octane Mixture Quantity X 300 x+300 % Isooctane 88% 92% 91% Amount of Isooctane 0.91 (Simplify your answers. Use integers or decimals for any numbers in the expression.)arrow_forwardThe following chemical reaction is an/a _______ reaction? 2C2H5OH(l) + 7O2(g) → 4CO2(g)+ 6H2O(g)+ 654 kcal Exothermic or endothermic?arrow_forwardb) HO OHarrow_forward
- Br 01 HNMe2 SN1 SN2 E1 E2 No Reactionarrow_forwardBr SH Heat Elarrow_forwardCopper can be drawn into thin wires. How many meters of 34–gauge wire (diameter=6.304 × 10 in) can be produced from the copper in 5.00 lb of covellite, an ore of copper that is 66.0% copper by mass? (Hint: Treat the wire as a cylinder: Vof cylinder = arh; d of copper=8.95 g/cm*). %3D !!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div