
World of Chemistry, 3rd edition
3rd Edition
ISBN: 9781133109655
Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher: Brooks / Cole / Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
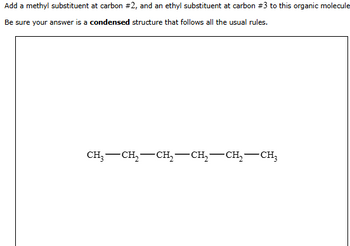
Transcribed Image Text:Add a methyl substituent at carbon #2, and an ethyl substituent at carbon #3 to this organic molecule
Be sure your answer is a condensed structure that follows all the usual rules.
CH3-CH2-CH₂-CH2-CH₂-CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Add a methyl substituent at carbon #2, and an ethyl substituent at carbon #4 to this organic molecule. Be sure your answer is a condensed structure that follows all the usual rules. CH₂ CH₂ CH₂ CH₂ CH₂ CH₂ CH₂ - C P :U G D tu 1)arrow_forwardWould u help mearrow_forwardRewrite each of these structures as a condensed structure. H F\H H H-C-Ċ-Ċ –Ċ-H H FHH condensed structure: CH3CF2CH2CH3 H H нНН H. H-С—С—С—Н H O H. 1 H. condensed structure: CH-3-CH-CH3arrow_forward
- Draw the complete structural formula from each condensed structure. Include all hydrogen atoms.arrow_forwardI have given you a condensed structure. You need to convert it to an accurate bond-line structure. CH3 CH3-CH-CH2-CH-CH₂-C-H 12-CH-CH2₂-C-1 CI Draw (as bond-line structures) isomers of this compound where you only move the chlorine atom. Draw four isomers of the original compound that would have a five carbon chain as the longest chain. [Note: there would be many isomers that will satisfy this. Find any four.] Using the original molecule (and looking at the carbon next to the aldehyde carbonyl) what would be the charge on that carbon if I removed an H atom and left behind the pair of electrons? Circle the best answer Positive Negative Neutral In the space below, draw that structure (from the sentence above) as a bond-line structure. Then, draw a resonance structure for this ion and be sure to add curved arrows to show the movement of electrons.arrow_forwardanswer the 3 questions pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning