
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
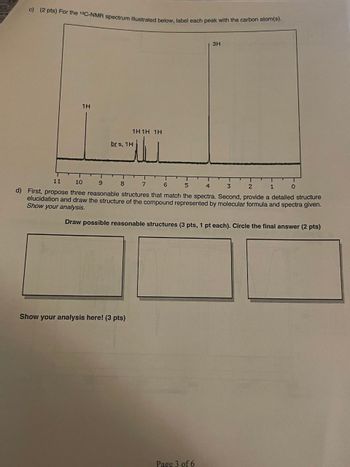
Transcribed Image Text:c) (2 pts) For the 13C-NMR spectrum illustrated below, label each peak with the carbon atom(s).
1H
brs, 1H
1H1H 1H
ــلتـ
3H
11
10
9 8
7 6 5
4
3
2
1
0
d) First, propose three reasonable structures that match the spectra. Second, provide a detailed structure
elucidation and draw the structure of the compound represented by molecular formula and spectra given.
Show your analysis.
Draw possible reasonable structures (3 pts, 1 pt each). Circle the final answer (2 pts)
Show your analysis here! (3 pts)
Page 3 of 6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Compound 2 has molecular formula C6H12. It shows three signals in the 1H-NMR spectrum, one at 0.96 ppm, one at 2.03 ppm, and one at 5.33 ppm. The relative integrals of these three signals are 3, 2, and 1, respectively. Provide structure for compound 2, explain how you reached your conclusion.arrow_forward1Compound 1 has molecular formula C7H16. It shows three signals in the 1H-NMR spectrum, one at 0.85 ppm, one at 1.02 ppm, and one at 1.62 ppm. The relative integrals of these three signals are 6, 1, and 1, respectively. Compound 2 has molecular formula C7H14. It shows three signals in the 1H-NMR spectrum, one at 0.98 ppm, one at 1.36 ppm, and one at 1.55 ppm. The relative integrals of these three signals are 3, 2, and 2, respectively. Propose structures for compounds 1 and 2, explaining how you reach your conclusion.arrow_forwardCompound 1 has molecular formula C7H16. It shows three signals in the 1H-NMR spectrum, one at 0.85 ppm, one at 1.02 ppm, and one at 1.62 ppm. The relative integrals of these three signals are 6, 1, and 1, respectively. Compound 2 has molecular formula C7H14. It shows three signals in the 1H-NMR spectrum, one at 0.98 ppm, one at 1.36 ppm, and one at 1.55 ppm. The relative integrals of these three signals are 3, 2, and 2, respectively. Propose structures for compounds 1 and 2, explaining how you reach your conclusion.arrow_forward
- Compound 1 has molecular formula C7H15Cl. It shows two signals in the 1H-NMR spectrum, one at 1.08 ppm and one at 1.59 ppm. The relative integrals of these two signals are 3 and 2, respectively. Propose structures for compound 1, explaining how you reach your conclusion.arrow_forwardA 13C NMR spectrum is shown for a molecule with the molecular formula of C4H8O2. Draw the structure that best fits this data.arrow_forwardDetermine the structure of a compound with the formula C11H15ClO2 based on the 1H NMR spectrum below. Based on its 13C NMR (DEPT) spectrum, it is evident that this molecule shows 9 signals including one methyl (CH3) and four methylene (CH2) units.arrow_forward
- The 'H NMR spectrum of compound A (C3H100) has four signals: a multiplet at 8 = 7.25-7.32 ppm (5 H), a singlet at d = 5.17 ppm (1 H), a quartet at d = 4.98 ppm (1 H), and a doublet at ô = 1.49 ppm (3 H). There are 6 signals in its 13C NMR spectrum. The IR spectrum has a broad absorption in the -3200 cm-1 region. Compound A reacts with KMNO4 in a basic solution followed by acidification to give compound B with the molecular formula C7H6O2. Draw structures for compounds A and B.arrow_forwardA ¹H NMR spectrum is shown for a molecule with the molecular formula of C10H12O4. Draw the structure that best fits this data. 1H 11 10 2H 2H U 7 1H 1H ΤΗ 1Η ppm Qarrow_forwardA'H NMR spectrum is shown for a molecule with the molecular formula of CeH9Br. Draw the structure that best fits this data.arrow_forward
- Compound 1 has molecular formula C6H12. It shows three signals in the 1H-NMR spectrum, one at 0.96 ppm, one at 2.03 ppm, and one at 5.33 ppm. The relative integrals of these three signals are 3, 2, and 1, respectively. Compound 2 has molecular formula C7H15Br. It shows two signals in the 1H-NMR spectrum, one at 1.08 ppm and one at 1.59 ppm. The relative integrals of these two signals are 3 and 2, respectively. Propose structures for compounds 1 and 2, explaining how you reach your conclusion.arrow_forwardCompound C has molecular formula C5H8O. The IR, mass, 1H-NMR, and 13C-NMR spectra are shown below. Suggest a structure for C and explain your reasoning.arrow_forwardThe H1H1 NMR spectra corresponds to an alcohol with the molecular formula C5H12O. Deduce the structure from the spectraarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
