
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
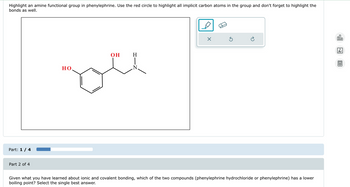
Transcribed Image Text:Highlight an amine functional group in phenylephrine. Use the red circle to highlight all implicit carbon atoms in the group and don't forget to highlight the
bonds as well.
Part: 1/4
Part 2 of 4
OH
H
но.
N.
☑
Given what you have learned about ionic and covalent bonding, which of the two compounds (phenylephrine hydrochloride or phenylephrine) has a lower
boiling point? Select the single best answer.
000
18
Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 1. which IR stretches and bends indicate what type of functional group we have (alcohol, carboxylic acid, ester), 2. how you can then use IR to monitor the change from the starting materials to the products. 3. indicate and discussion the absorptions that are relevant to alcohol OH and C-O, carboxylic acid OH, carboxylic acid C=O, ester C=O, and C-O). the picture below is an ir spectra of ethanolarrow_forwardPlease step by step answer and don't use ASI Answerarrow_forwardGive the omega-n designation for each acid. Part 1 of 2 oleic acid CH3(CH2), CH=CH(CH2), COOH omega- acid ☑ Part 2 of 2 paullinic acid CH3(CH2), CH=CH(CH2) COOH 5 11 omega- acid Хarrow_forward
- With respect to the parent compound, explain in detail how modifying the rings shown as A, B, C, D and E in morphine can affect the biological activity associated with the moleculearrow_forward3). reactions of the following alkyl iodide. Danny uses potassium tert-butoxide to promote the reaction while Claire uses water and high temperatures. One of their reactions has provided a single elimination isomer while the other has provided a mixture of elimination isomers. Organic chemistry students Danny and Claire are both performing elimination Me H20, heat Danny's Reaction Claire's Reaction H. Me a) Which student's reaction (Danny or Claire) has provided a single elimination isomer? b) Draw all the elimination isomers obtained from both students' reactions. c) Which of the dienes drawn (by you) in question 3b is the most stable? Circle that diene above.arrow_forwarddraw the alkane strucutre based off the IR spectrum, 1H NMR spectrum, and 13C NMR spectrum for this compound.arrow_forward
- 10. Glycine has 2 ionizable functional groups. Write the equilibrium equations for its three ionizations, and assign the proper pKa (2.34, 9.60) for each ionization. Draw the structure of Glycine in three ionization states. Indicate the net charge on the Glycine molecule in each ionization state. Structure Net charge a. COO- H3N-C-H H Please calculate the isoelectric point of Glycine. b. Draw the structures of the predominant ionization state of Glycine at pH 1 and 14.arrow_forwardDraw the major organic product for each of the following reactions. You must show the correct stereochemistry of the product where relevant.arrow_forwardDraw the structure of the product that forms when the carbonyl compound shown is treated with K2Cr2O7. If no reaction occurs, draw the structure of the organic starting material (reactant). CHOarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting structure, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Then draw any missing organic intermediates or products for this epoxidation reaction. Include all lone pairs in the structures. Ignore inorganic byproducts, counterions, and solvents. 0. ~ H :OH: H Q H --0 Q mCPBA dilute HCI Select to Draw Intermediate 0:0 NaOH :OH: H Select to Add Arrows H I I I I I I I I I Iarrow_forwardOrganic chemistryarrow_forwardWrite a sample chemical reaction of chymotrypsin in a complete balanced equation label the following properly: substrate/s, cosubstrate/s, and cofactor/s Show all the changes in the reacting components small molecules should be in their skeletal form large molecules could be shown as hybrid structures - reacting/interacting groups in skeletal form, the rest of the molecule as abbreviations/blocks/shapesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON