
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
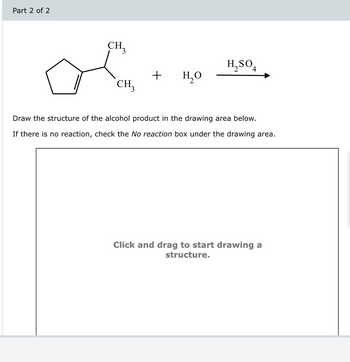
Transcribed Image Text:Part 2 of 2
CH₁₂
H2SO4
+
но
CH3
Draw the structure of the alcohol product in the drawing area below.
If there is no reaction, check the No reaction box under the drawing area.
Click and drag to start drawing a
structure.
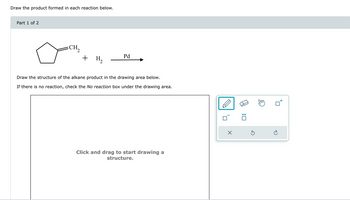
Transcribed Image Text:Draw the product formed in each reaction below.
Part 1 of 2
CH₂
+ H₂
Pd
Draw the structure of the alkane product in the drawing area below.
If there is no reaction, check the No reaction box under the drawing area.
Click and drag to start drawing a
structure.
:
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- A scientist synthesized an unknown compound. It is soluble in sulfuric acid but not water. They then took a proton NMR using deuterated chloroform. The results are shown below. Identify the unknown compound using the data provided. Bonus: What is the IUPAC name of this compound?arrow_forwardModify the structure to show the MAJOR product that would be formed in the following reaction. HNO3, H2SO4arrow_forwardWhat is the wavelength of a 6.76 x 1012 /s wave? Sorry im just really stuck on this question, Please dont leave any minor detail in the question, And also may u step by step this equation.arrow_forward
- Draw structural formulas for the alkoxide ion and the alkyl(aryl)bromide that may be used in a Williamson synthesis of the ether shown. CH3CH2CH2CH2-0-CH2CH2CH2CH3 • You do not have to consider stereochemistry. • Do not include counter-ions, e.g., Na*, I, in your answer. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. O Separate structures with + signs from the drop-down menu.arrow_forwardIdentify the functional groups in the following molecule as pointed by arrow A and B, then C and Darrow_forwardDraw the structure of the product that forms when the carbonyl compound shown is treated with K₂ Cг2 О7. If no reaction occurs, draw the structure of the organic starting material (reactant). CH3(CH2), (CO)CH (CH3)2 Click and drag to start drawing a structure. : ☐arrow_forward
- Identify the names of the following molecules.arrow_forwardPLEASE SHOW ALL DETAILED WORKarrow_forwardPart A For the reaction Br2 (1) 2 Br (g) at what temperature is the equilbrium constant equal to 3.5? t> VO ΤΙ ΑΣΦ 0 ? Your answer should not contain commas. No credit lost. Try again. Submit Previous Answers Request Answer Karrow_forward
- Suggest to biochemistryarrow_forwardUsing the bond dissociation energy table from Brightspace, calculate the enthalpy change of the following reaction: HO. + HBr Please enter your response. Submit Br + H₂Oarrow_forwardDraw a curved arrow mechanism for the reaction, adding steps as necessary. Be sure to include all electrons that are necessary to the mechanism and all nonzero formal charges. о :0- 0-н .0. + Add/Remove step Click and drag to start drawing a structure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON