
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
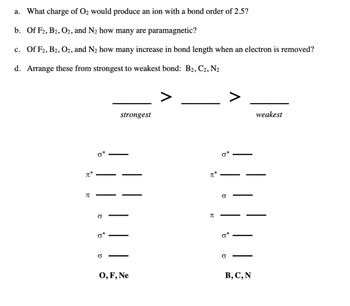
Transcribed Image Text:a. What charge of O2 would produce an ion with a bond order of 2.5?
b. Of F2, B2, O2, and N2 how many are paramagnetic?
c. Of F2, B2, O2, and N2 how many increase in bond length when an electron is removed?
d. Arrange these from strongest to weakest bond: B2, C2, N2
π*
Π
b
9
σ
strongest
O, F, Ne
བུ
π*
π
b
σ*
σ
weakest
B, C, N
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Select the sentence that correctly describes the relationship between this pair of molecules. a They are both enantiomers and isomers. b They are enantiomers but not isomers. c They are isomers but not enantiomers. d They are neither enantiomers nor isomers.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting structure, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Then draw any missing organic intermediates or products for this epoxidation reaction. Include all lone pairs in the structures. Ignore inorganic byproducts, counterions, and solvents. 0. ~ H :OH: H Q H --0 Q mCPBA dilute HCI Select to Draw Intermediate 0:0 NaOH :OH: H Select to Add Arrows H I I I I I I I I I Iarrow_forward1. Polar Covalent Bonds a. Draw one water molecule. b. Indicate which bonds are polar covalent bonds by using a solid red line for each of those bonds. c. Label the partial charges.arrow_forward
- What is the relationship between the following pair. CH,Br CI CH3 CI CH2CH3 Но HOl.. CH,Br CH2CH3 CH3 Enantiomers Diastereomers Conformers O Identical O Constitutional isomersarrow_forwardWhat kind of bond is shown in letter B? (Select the best answer) А. Adenine B.- Cyne Guanne Thymane С. D.arrow_forwardFor the following reaction at equilibrium NH3 + H20 + NH4+1 + Он-1 NH3 is the It is not a hydroxide and so is a base. H20 is the It is both a very acid and a very base. NH4*1 is the It is not on the list of strong acids and so is a acid. OH-1 is the . It is a base because it contains hydroxide. а. acid b. base c. conjugate acid d. conjugate base e. strong f. moderately strong or weak g. weakarrow_forward
- 1. Draw a circle around and give the name of each functional group in the following twomolecules. (Note: each line represents a covalent bond. If no atom is shown, assume there’s a carbon there.)arrow_forwardD Question 3 Observe these two structures and choose the correct statement Но Но A and B have the same boiling point O A is more commonly found in nature compared to B O A and B are isomers O A and B are PUFA « Previousarrow_forward6. Proteins contain other amino acids that can compete with cysteine and react with bromoacetamide. Compare the structure of cysteine and serine below. Why can serine react similarly to cysteine? Think about periodic table trends that influence electronic factors. SH I H N H cysteine Brief explanation of why serine can react similarly to cysteine: serine OHarrow_forward
- It's about chemical bonding I am a bit confuse of how to figure out what is a anion or a cationarrow_forward12. What is the pH of a solution in which [OH] = 0.000005 M? )5.3 8.7 O 5 x10arrow_forward3. Reactants A-B and B-C react according to the equation below. In what orientation must the reactant particles collide in order to be effective? Be sure to draw the collision (activated complex), and use a dotted line for any bonds being made or being broken. A-B + В-С → A-C 2 Barrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON