
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
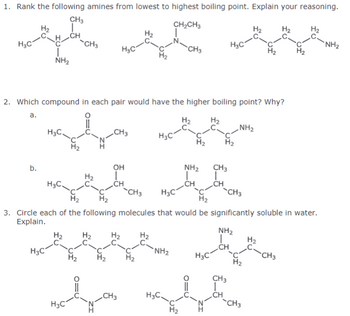
Transcribed Image Text:1. Rank the following amines from lowest to highest boiling point. Explain your reasoning.
CH3
CH2CH3
H3C
CH3
༣.མིའི་ན་བྱིན་
CH
NH₂
-CH3
H₂
NH2
2. Which compound in each pair would have the higher boiling point? Why?
a.
H3C
CH3
H3C
OH
b.
NH2
CH3
H₂
H3C
CH
CH
CH3
H3C
-CH3
NH2
3. Circle each of the following molecules that would be significantly soluble in water.
Explain.
ΝΗΣ
H₂
H₂
H₂
H₂
CH
C-
NH₂
H3C
-CH3
H3C
H2
H₂
CH3
CH
CH3
H3C
-CH3
H3C
H₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 1 steps with 3 images

Knowledge Booster
Similar questions
- A sample was analyzed using mass spectrophotometer and molecular ion peak was obtained at m/z 120. Base peak was seen at m/z 91 and another peak of was observed at m/z 92. UV spectroscopic analysis revealed the presence of an aromatic ring. Determine the compound and justify your answer. And draw the mass spectrum.arrow_forwardGive the IUPAC name for each compound. Part 1 of 4 H,C HO H -CH,CH,CH, 2-Pentanol ྾ 6 Part 2 of 4 OH H;C- C—CH(CH,CH,CH; H 2 ྾ ཅarrow_forwardThis is my question .arrow_forward
- I need the answer as soon as possiblearrow_forward1. Which are aromatic? H-N I-N I II III IV (A) I and III (C) II and III (B) II and IV (D) I and II 2. Rank these radicals from least to most stable. I II III (A) Iarrow_forwardDraw the major organic product for each of the following reactions. You must show the correct stereochemistry of the product where relevant.arrow_forward46 2 H G CI 8 Is M (n Ge M In VI Q LE Q S M In ь м + uv A canvas.northseattle.edu/courses/2086259/quizzes/5876542/take CH,OH Hc-OH HOc-H H c-OH он OH он HC-OH Account OH H C-OH H Dashboard This image shows two configurations of the same molecule,a(n) [ Select ] v, and would primarily exist like the molecule on the Courses [ Select ] within a living cell. Groups This molecule is [ Select ] , and [ Select ] Calendar dissolve in water. Inbox History 3 •i 8:14arrow_forward5. Assign the IUPAC name for the following amines (15pts) C2H5 CH3-CH-CH-CH,-CH,CH2-CH2-CH3 a. b. CH3-CH2-CH2-NH-CH3 NH2arrow_forwardClessify the alcohol with the following structure: NHCOCH, CH,OH Select one: a. none of the other answers Ob. primary E secondary d. tertiary e. quaternaryarrow_forwardHow do you prepare 42:1 chloroform/isoamyl alcohol 70% ethanol?arrow_forwardA scientist synthesized an unknown compound. It is soluble in sulfuric acid but not water. They then took a proton NMR using deuterated chloroform. The results are shown below. Identify the unknown compound using the data provided. Bonus: What is the IUPAC name of this compound?arrow_forwardWhat is the pH of a cleaning solution with a [H3O*] = 7.4 x 10-10 M H3O*? A. 7.4 B. 9.00 C. 8.13 D. 9.13 E. 7.13arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON