
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
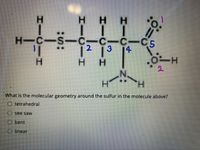
Transcribed Image Text:H H
H-C-S-C-C-C-C5
12 13
14
H H
2.
H.
What is the molecular geometry around the sulfur in the molecule above?
O tetrahedral
see saw
bent
linear
HICIN:
HICIH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- property that will be different in the followin 1) Identify One geometry iso-electronic molecules (iso-electronic compounds based on electrons). molecolar have the same number of valence a) (F. Br and Cfrr 4 b) OCS and CO₂ # dias 1 Sport 10 HA L P dowarrow_forwardHHHO. H-C-S-C-C-C C-C5 1² ]³ 2 3. 4 H. - 2. H. H. Which carbons have a tetrahedral molecular geometry in the molecule above? O 1,2, & 3 O 1,2, 3, & 5 All of them O 1,2, 3, & 4 HICIIarrow_forwardHow many o and a bonds are in this molecule? H. 0-H H-C C-H H. H number of o bonds: number of r bonds: 939 PM 10/27/2020 DELLarrow_forward
- 5-54 Using VSEPR theory, predict the molecular geometry of the following molecules. a. :0: :0::N:0:H b. O: H H:C:C:H Harrow_forwardplease do d, e and f the last 3arrow_forwardConsider the following molecule: Which orbitals overlap to form the carbon-oxygen sigma bond of this molecule? H2CCH2O(1).jpg| see attached image con es) O Csp and Osp suc O Csp? and 02p raries O Csp? and Osp O Csp and N2p al ey O Csp and Osp3 ery O N2p and C2p C2p and C2p O Nsp³ and Csp? 10 pts Ouestion 2arrow_forward
- 4. C/ 4 8:4 EG:tetrahedre NB:0 MGitetnatedrel EG: tetralleira 0:2 MG bent NB: 2 4 central (CH₂CH₂) polar or nonpolar? Explain. Potor, because Chorine has by higher electrones & Niets St then hydrogeme which results alim in uneque palling palling ond since it's also not symmetrica. atom? How many "central" atoms are part of methanol (CH3OH)? What are the molecular geometries of each central 2 Centro otorns H-0₁- atoms H B: 2 - NB: 2 MG bert I-N - 5. How many "central" atoms are part of ethylene glycol (HOCH₂CH₂OH), a component of antifreeze? What are the molecular geometries of each central atom? Is the molecule polar or nonpolar? Why? (Note: the modeling kits technically only contain one carbon and one oxygen via color coding, however, in this example, other spheres can be used to represent these elements within this molecule why?) 4 I U-I ... Or Ch 9-12 EG: tetrahedro B:4 66% terrehend NB:0 MG: teaters Parrow_forwardWhich of the following is the correct line bond structure for the following Newman projection? EL B. D. OD O B O A Question 2arrow_forwardConsider the structure below. What is the molecular geometry for the circled central atom? H. H H H H H. -CEC-Harrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY