
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
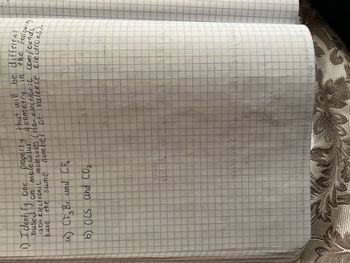
Transcribed Image Text:property that will be different
in the followin
1) Identify One
geometry
iso-electronic molecules (iso-electronic compounds
based on
electrons).
molecolar
have the same
number of valence
a) (F. Br and Cfrr
4
b) OCS and CO₂
#
dias
1
Sport
10
HA
L
P
dow
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 20) For carbon disulfide (toxic, malodorous, flammable liquid) and selenium disulfide (solid, melting point 111oC, over-the-counter drug used to treat certain types of dermatitis) a) compile their Lewis electron dot formulas b) identify their electron pair geometry according to the VSEPR theory, c) determine which of these two molecules is polar and which is non-polar and shortly explain whyarrow_forwardThe ion PO 4 A/ 3- has double bonds. A 1 total valence electrons, the shape is , and the molecule containsarrow_forward4. Explain why the following molecules can NOT existis Xarrow_forward
- Use the information on the fictious nonmetal elements. to answer the following Element Colomn EN ff 1.9 14 17 3.1 2.3 3.6 15 Nn 16 Shoos one of the followng mokcular compounds and completc the queSTIons: Nn Oyzlshape is benti V) ODraw a leuis Structure Shoudng all lalonce electrons. ) Calculate the electrongativity the bond. State what a paar covalert band, notate the partial difference for Gf bond will form If it is charges d) Detamine if the molecule is polar or polar. non-arrow_forwardPredict the electron pair geometry and molecular geometry for the following substances by drawing their Lewis structures. Also state if each substance is polar or nonpolar. a) BH3. i) Lewis Structure: b) H₂O ii) Electron pair Geometry: iii) Molecular Geometry: iv) Polar or Nonpolar:arrow_forward13.) All of the following follow the octet rule EXCEPT Group of answer choices NBr3 BrCl SCl2 BeCl2 CHCl3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY