
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
can i get help with these problems
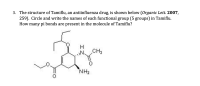
Transcribed Image Text:3. The structure of Tamiflu, an antiinfluenza drug, is shown below (Organic Lett. 2007,
259). Circle and write the names of each functional group (5 groups) in Tamiflu.
How many pi bonds are present in the molecule of Tamiflu?
H.
CH3
N.
NH2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- thx!arrow_forwardChemistry Determine the number of electrons that are be capable of delocalizing. :O: :F: O 8 O 12 O 4 O 2 Which set (or sets) of molecules shows two isomers? I. H-ö: II. H H. `C C H. H .O. III. H :Br: IV. H :Br: :Br: H H :Br -с —с- н н-с—с-—Н H H H :Br: O Set III shows a pair of isomers O Sets III and IV both show pairs of isomers O Set I shows a pair of isomers O Set Il shows a pair of isomers O Sets I and IIl both show pairs of isomers Lattice energy relates to which of the following chemical and/or physical properties? O electrolyte strength O acid strength O combustibility O The temperature at which a molecular compound boils エ エarrow_forwardhelparrow_forward
- Chemistry [Hint: Carbon atoms in line diagrams are where bond lines meet and are not always written. This is similar to the exercise in the textbook.] Shown below is the structure of a polypeptide. There are [Select] and [Select] H₂N peptide bonds in this polypeptide, represents the peptide bond. NH NH₂ -NH₂arrow_forwardDraw an orbital picture of propene. The bond between and is formed by the overlap of which types of orbitals? a. sp2 and sp2 b. sp3 and sp3 c. sp2 on Ca and sp3 on Cb d. sp3 on Ca and sp2 on Cb The double bond between and is formed by what? a. two sigma bonds b. two pi bonds c. overlap of a p orbital on Cb with a sp2 orbital on Cc. d. overlap of two p orbitals plus overlap of two sp2 orbitalsarrow_forward. Draw the Lewis diagram for acetamide. ( CH3CONH2) a. What is the electron geometry around each of the two carbon atoms. b. What is the hybridization type of each of the carbon atoms. c. What are the bond types and orbitals involved in bonding for all bonds between the C and O atoms.arrow_forward
- 6arrow_forwarda. Predict the molecular structure and bond angles for SeCl6. Approximate bond angles are sufficient. Molecular Structure = Bond Angles = b. Predict the molecular structure and bond angles for SeO2. Approximate bond angles are sufficient. Molecular Structure = Bond Angles =arrow_forwardQ. 50. The antibiotic tetracycline's structure is depicted below in the blue box. What is the number of sp3(C)-sp2(C) bonds present in it? There is only one right answer. a) 5 b) 11 c) 4 d) 8 OH O HQ, HO Ho H O NH₂ OHarrow_forward
- Which structure BEST describes the polarity of an H-O bond? a. + H-O Ob Ос O a b. 8- 8+ H-O C. &+ 8- H-Oarrow_forward3arrow_forwardNumber of Molecule valence electrons Formal Charge Electron-Group Geometry Molecular Geometry Resonance HSO4 S: S: Select one . S: Select one .. Select one XeTe4 Хе: Xe: Select one ... Xe: Select one .. Select one PHO P: P: Select one ... P: Select one .. Select one ... v PCIO P: P: Select one ... P: Select one .. Select one ..arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning