
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
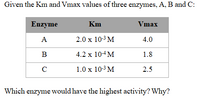
Transcribed Image Text:Given the Km and Vmax values of three enzymes, A, B and C:
Enzyme
Km
Vmax
A
2.0 x 10-3 M
4.0
B
4.2 x 104M
1.8
C
1.0 x 10-3 M
2.5
Which enzyme would have the highest activity? Why?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Do not give handwriting solution.arrow_forwardConsider the following experimental data from another experiment: [S] 1.5 2.00 2.50 5.00 10.00 V (No inhibitor) mmol ml¹ min¹ 0.167 0.204 0.232 0.313 0.385 V (inhibitor) mmol ml¹¹ min¹¹ 0.115 0.143 0.167 0.250 0.333 Calculate Km and V max and determine whether this inhibitor is competitive, non-competitive or uncompetitive.arrow_forwardYou are working on an enzyme that obeys standard Michaelis-Menten kinetics. You have determined the Vmax to be 0.1 mol/sec and the Km to be 2.5 mM. What would the rate of the reaction be when the substrate concentration is 20 mM? 0.09 MS-1 O 0.133 Ms-1 O 0.18 Ms ¹ 9 Ms-1 O 0.018 Ms-1 0.2 MS-1arrow_forward
- A particular reaction has a ΔG‡ of 37.0 kJ mol-1. In the presence of an enzyme, the same reaction has a ΔG‡ of 5.70 kJ mol-1. Calculate the value of ΔΔG‡ in kJ mol-1.arrow_forwardThe kinetic data of an enzymatic reaction shown in the following table: a. Calculate the Km and Vmax of the reactionb. A competitive inhibitor is added to the reaction at a concentration of 5 μM, determine the new Vmax of the reaction and what happened to the Km value.arrow_forwardThe enzyme triosephosphate isomerase (TIM) catalyzes the following reaction in glycolysis, where it converts dihydroxyacetone phosphate (DHAP) to glyceraldehyde-3-phosphate (GAP). CH₂OH C=O CH₂OPO²- DHAP triose phosphate isomerase [DHAP] (MM 1.00 2.00 3.00 6.00 O V. (mM/s) 3.700 6.727 9.250 14.800 = H HCOH CH₂OPO²- Kinetics experiments were performed on TIM. Enzyme activity (initial velocity, V.) was measured at varying concentrations of DHAP. The enzyme kinetics were also measured in the presence of two inhibitors, A and B. The enzyme concentration used in all experiments was 25.00 μM. The data are shown below. GAP V. (mM/s) + A 1.452 2.794 4.038 7.281 V₁ (mM/s) + B 0.755 1.379 1.905 3.077arrow_forward
- (b) You are investigating the effects of several agents on the activity of alcohol dehydrogenase. The enzyme activity data are shown in the table below. Construct a [substrate] vs. activity plot and a double-reciprocal plot for this enzyme. Be sure to label all axes. Determine the Vmax and KM for AD from the graphs in each type of plot. AD activity (nM/min) AD activity + agent A (nM/min) AD activity + agent B (nM/min) [Alcohol] (nM) 0.1 14 2 0.5 50 7 8. 1.0 65 10 30 2.0 72 12 45 4.0 80 14 62 8.0 85 15 75 32.0 90 16 90arrow_forwardAn enzyme catalyzes a reaction with a Km of 7.50 mM and a Vmax of 2.90 mM - s-1. Calculate the reaction velocity, un, for each substrate concentration. [S] = 2.75 mM mM · s- * TOOLS x10 [S] = 7.50 mM mM · s-! [S] = 11.0 mM mM - s-arrow_forwardver is given. A +B C+ D 1) Calculate AG for the above react un and indicáte whether the reaction is favorable or unfavorable [A] = 0.9 M 20°C AG° = 4 KJ/mol %3D [B] = 15mM [C] = 4mM [D] = 3 M R= 8.314 J/moleK %3D %3D 4.7 and explain how you know.arrow_forward
- Consider the Michaelis-Menten enzymes below and answer the following questions. Kcat (s') 9.5*105 1.4*10* 2.5*102 1.0*107 5.0*10 8.0*10² Enzyme Km (M) A В a. Which enzyme has the highest affinity substrate? How do you know? b. Which enzyme can convert the most substrate to product in a given period of time? How do you know? c. Which enzyme has the highest catalytic efficiency? How do you know?arrow_forwardHow many net molecules of nucleoside triphosphate (ATP and equivalent molecules) are produced by complete aerobic catabolism of a glucose going through glycolysis, the pyruvate dehydrogenase complex and the citric acid cycle (TCA cycle)? Do not count the ATP eventually generated by re-oxidation of reduced coenzymes, just the number of NTPs produced in reactions of these pathways. Choose the one best answer. 02 03 04 05 06 8arrow_forwardBelow is kinetic data obtained for an enzyme-catalyzed reaction. The enzyme concentration is fixed at 100 nM. Using a Lineweaver-Burke plot, calculate the catalytic efficiency for this reaction. Report your answer in scientific notation to three significant figures in units of M-1s-1.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON