
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
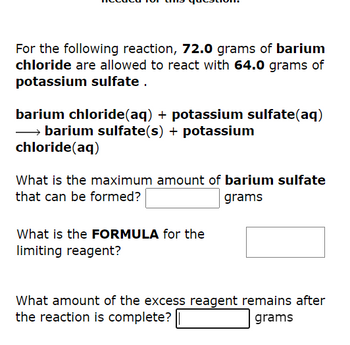
Transcribed Image Text:For the following reaction, 72.0 grams of barium
chloride are allowed to react with 64.0 grams of
potassium sulfate.
barium chloride(aq) + potassium sulfate(aq)
→→→→→barium sulfate(s) + potassium
chloride (aq)
What is the maximum amount of barium sulfate
that can be formed?
grams
What is the FORMULA for the
limiting reagent?
What amount of the excess reagent remains after
the reaction is complete?
grams
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The total acidity in water samples can be determined by neutralization with standard sodium hydroxide solution. What is the total concentration of hydrogen ion, H+, present in a water sample if 200. mL of the sample requires 7.7 mL of 2.8 ✕ 10-3 M NaOH to be neutralized?arrow_forwardPrecipitation reactions can be used to extract elements, such as magnesium from seawater. Using the solubility table, determine which of the following solutions could be used to precipitate out the magnesium ions from a sample of seawater? O AGNO3(aq) O NazCO3(aq) O KCl(aq) O NH4Br(aq)arrow_forwardMagnesium metal reacts with hydrochloric acid to form aqueous magnesium chloride and hydrogen gas. When 7.30 g of magnesium metal is added to 100.0 mL of 3.00 M hydrochloric acid solution, what mass of hydrogen gas is produced, assuming the reaction goes to completion? a) Not enough information is provided. b) 0.302 g c) 0.605g d) 0.247 g e) 0.151 garrow_forward
- Barium carbonate is the source of barium compounds. It is produced in an aqueous precipitation reaction from barium sulfide and sodium carbonate. (Barium sulfide is a soluble compound obtained by heating the mineral barite, which is barium sulfate, with carbon.) What are the molecular equation and net ionic equation for the precipitation reaction? A solution containing 33.9 g barium sulfide requires 21.2 g of sodium carbonate to react completely with it, and 15.6 g of sodium sulfide is produced in addition to whatever barium carbonate is obtained. How many grams of barium sulfide are required to produce 500 tons of barium carbonate?arrow_forwardWhat type of chemical reaction is illustrated in the following example? N2(g) + H2(g) → NH3(g) A) combination reaction B) decomposition reaction C) single-replacement reaction D) double-replacement reaction E) neutralization reaction What type of chemical reaction is illustrated in the following example? Zn(s) + HCl(aq) → ZnCl2(aq) + H2(g) A) combination reaction B) decomposition reaction C) single-replacement reaction D) double-replacement reaction E) neutralization reaction What type of chemical reaction is illustrated in the following example? AlCl3(aq) + AgNO3(aq) → Al(NO3)3(aq) + AgCl(s) A) combination reaction B) decomposition reaction C) single-replacement reaction D) double-replacement reaction E) neutralization reactionarrow_forwardA 0.8910 g sample of a mixture of NaCl and KCl is dissolved in water, and the solution is then treated with an excess of AgNO3 to yield 1.923 g of AgCl. Calculate the percent by mass of each compound in the mixture. Be sure each of your answer entries has the correct number of significant digits. % mass NaCl 0% % mass KCI Xarrow_forward
- In the laboratory, a student dilutes 25.3 mL of a 8.46 M hydrobromic acid solution to a total volume of 100.0 mL. What is the concentration of the diluted solution?arrow_forwardFor the following reaction, 6.80 grams of water are mixed with excess chlorine gas. The reaction yields 15.1 grams of hydrochloric acid. chlorine (g) + water (1) →→→→hydrochloric acid (aq) + chloric acid (HCIO3) (aq) What is the theoretical yield of hydrochloric acid ? || What is the percent yield of hydrochloric acid ? grams %arrow_forwardIn an experiment, a student combines 75.0 mL of a 0.190 M iron (III) chloride solution with 125.0 mL of a 0.240 M sodium carbonate solution. What is the theoretical yield of sodium chloride (in grams)?arrow_forward
- In an experiment, a student combines 75.0 mL of a 0.200 M iron (III) chloride solution with 125.0 mL of a 0.250 M sodium carbonate solution. Write a balanced equation for the reaction. What is the limiting reactant? What is the theoretical yield of sodium chloride (in grams)? How many grams of sodium chloride need to be produced in the experiment in order to achieve a yield of 93.75%?arrow_forwardThe concentration of a certain sodium hydroxide solution was determined by using the solution to titrate a sample of potassium hydrogen phthalate (abbreviated as KHP). KHP is an acid with one acidic hydrogen and a molar mass of 204.22 g/mol. In the titration, 48.82 mL of the sodium hydroxide solution was required to react with 0.7540 g KHP. Calculate the molarity of the sodium hydroxide. Molarity = ___________ Marrow_forwardSolid copper can be produced by passing gaseous ammonia over solid copper (II) oxide at high temperatures, according to the following reaction. NH3 (g) + CuO (s) → N2 (g) + Cu (s) + H2O (g) Balance the reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY