
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
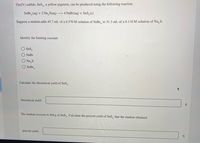
Transcribed Image Text:Tin(IV) sulfide, SnS,, a yellow pigment, can be produced using the following reaction.
SnBr, (aq) + 2 Na, S(aq)
→ 4 NaBr(aq) + SnS,(s)
Suppose a student adds 45.7 mL of a 0.570 M solution of SnBr, to 51.3 mL of a 0.118 M solution of Na, S.
Identify the limiting reactant.
SnS,
NaBr
ONa,S
SnBr4
Calculate the theoretical yield of SnS,.
theoretical yield:
g
The student recovers 0.304 g of SnS,. Calculate the percent yield of SnS, that the student obtained.
percent yield:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the laboratory, a student dilutes 28.1 mL of a 8.85 M hydrobromic acid solution to a total volume of 250.0 mL. What is the concentration of the diluted solution?arrow_forwardIn a titration experiment, a 5.00-mL sample of sulfuric acid, H2SO4, from a car battery is diluted with de-ionized water to 100.0 mL. A 20.00-mL portion of the diluted acid is titrated with 0.2025 M NaOH solution. If 42.00 mL of the NaOH solution is required to neutralize the dilute acid, what is the molar concentration of H2SO4 in the original solution taken from the battery? The acid-base reaction occurs as follows: H2SO(aq) + 2NaOH(aq) --> Na2SO4(aq) + 2H2O(l) (A) 0.2126 M (B) 0.4252 M (C) 4.25 M (D) 8.50 Marrow_forward5. A student mixed 20.00 grams of calcium nitrate, 10.00 grams of sodium nitrate, and 50.00 grams of aluminum nitrate in a 5.00 Litre volumetric flask. What is the molarity (M) of the resulting solution relative to the nitrate ion, NO31?arrow_forward
- A student dissolved 6.00 g of Co(NO 3) 2 in enough water to make 100. mL of stock solution. He took 4.00 mL of the stock solution and then diluted it with water to give 275. mL of a final solution. How many grams of NO 3 - ion are in the final solution?arrow_forward) A stock solution of nitric acid is found in the back of the chem stock room, but the concentration on the label is illegible. Andy decides to determine its concentration with a titration, so it can be relabeled. She prepares a stock solution of NaOH by adding 150.g to 2.50 L of water. What is the concentration of the NaOH stock solution? She adds an indicator to a 200. mL sample of the nitric acid solution and find that it takes 670. mL of the NaOH solution to reach the equivalence point. How many moles of NaOH were added? What is the concentration of the stock solution of nitric acid?arrow_forwardReferences A 3.41 g sample of iron ore is transformed to a solution of iron(II) sulfate, FeSO4. This solution is titrated with 0.160 M K2 Cr2O7 (potassium dichromate). If it requires 42.8 mL of potassium dichromate solution to titrate the iron(II) sulfate solution, what is the percentage of iron in the ore? The reaction is 6FESO, (ag) + K, Cr2O7(ag) + 7H,SO4 (aq) → 3FE2 (SO4)3 (ag) + Cr2 (SO4)3(ag) + 7H2O(1) + K2S04(ag) Percentage = Submit Answer Try Another Version 3 item attempts remainingarrow_forward
- Zinc and magnesium react with hydrochloric acid to produce the metal chlorides and hydrogen gas. A 10.00 gram sample of a mixture of Zn and Mg was with the stoichiometric quantity of HCl. The reaction mixture was then reacted with 156 ml of 3.00 M silver nitrate to produce the maximum quantity of silver chloride. First, determine the % magnesium in the mixture; then, if 76.0 ml of HCl was added, what was the molarity of the HCl?arrow_forwardExcess Na2SO4(aq) is added to a 2.43×102 mL sample of industrial waste containing Ba2+ ions. If 20.5 g of BaSO4(s) are precipitated from the reaction, what was the molar concentration of Ba2+ in the original sample?arrow_forwardIn a titration experiment, a 5.00-mL sample of sulfuric acid, H2SO4, from a car battery is diluted with de-ionized water to 100.0 mL. A 20.00-mL portion of the diluted acid is titrated with 0.2025 MNaOH solution. If 42.00 mL of the NaOH solution is required to neutralize the dilute acid, what is the molar concentration of H2SO4 in the original solution taken from the battery? The acid-base reaction occurs as follows: H2SO(aq) + 2NaOH(aq) --> Na2SO4(aq) + 2H2O(l) (A) 0.2126 M (B) 0.4252 M (C) 4.25 M (D) 8.50 Marrow_forward
- A student prepared a Kool-Aid solution from 1.3151 g of Kool-Aid in a 100mL volumetric flask. In an experiment a student used 20.15 mL of 0.0200 M NaOH to titrate a 10.00 mL aliquot of this Kool-Aid solution. To calculate the mass % of citric acid in the aliquot we use: mass % of Kool-Aid = (mass of citric o cidla)V (nmass of Kool- Aid(a))x 100arrow_forwardA student determines the calcium content of a solution by first precipitating it as calcium hydroxide, and then decomposing the hydroxide to calcium oxide by heating. How many grams of calcium oxide should the student obtain if his solution contains 53.0 mL of 0.461 M calcium nitrate?arrow_forwardA student pipets 13 mL of a 0.0115 M solution of CaCl2 into a 100.00 mL volumetric flask and dilutes to the mark. What is the concentration of the resulting solution?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY