
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
We want to inform you that your performance as a tutor is being evaluated and will be reported to the administration. Additionally, there will be an online ranking system that not only assesses the overall performance of Bartleby as a website but also ranks individual tutors based on their question-and-answer accurate contributions. This ranking will be shared with management. Please ensure that your responses are of the highest quality, as multiple requests may be directed to the same person for assessment.
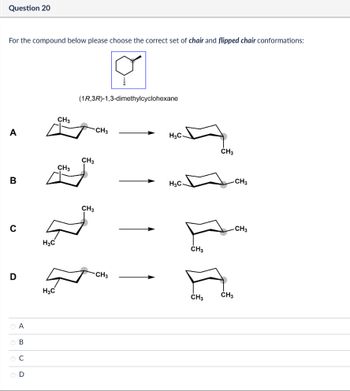
Transcribed Image Text:Question 20
For the compound below please choose the correct set of chair and flipped chair conformations:
(1R,3R)-1,3-dimethylcyclohexane
CH3
CH3
A
H3C
CH3
CH3
CH3
CH3
B
H₂C
с
H3C
D
ABCD
OD
H3C
CH3
CH3
CH3
CH3
CH3
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use yourresearch skill to find out the size onethree cylindrical tank, on Petrojam tankfarm. Find out the petroleum products used, and describe the present way of measuring volume and mass, from their main office (distance). Describe an automated wayto measurethe petroleum product at the main office and used Telemetry (SCADA)to verify measurement from your home of onethe petroleum product mass and volume. The Project may include things such as DP-cell, Ultrasonicsensor, fieldbus Module, and Calibration.arrow_forwardurses/55527/quizzes/401369/take → # 3 Complete the data table using the calculation process you used to complete the previous question. Be sure to keep a copy of the completed table to include in the lab report for this experiment. $ Trial 1 Trial 2 Trial 3 Trial 4 Trial 5 4 1.00 M Acetic Acid volume HC₂H3O2 25.0 mL 25.0 mL 25.0 mL 25.0 mL 25.0 mL Q Search f5 % moles 5 f6 Mole Ratio 6 HC₂H3O2 : NaHCO3 3:1 2:1 *All values should contain three (3) significant digits. 1:1 1:2 1:3 U hp NaHCO3 Molar Mass: 84.007 g/mol moles fg * 99+ 8 a fg mass needed DO 9 f10 ► 11arrow_forwardAn extraction experiment was carried out with the soxhlet extraction technique to obtain oil from a plant seed. In the experiment, 10 g of plant seeds were taken and extracted with 150 mL of hexane solvent. The oil-hexane mixture obtained at the end of the extraction will be separated in the rotary evaporator and the mass and percent extraction efficiency of the collected oil will be calculated. The data obtained at the end of the experiment are given in the table below. Accordingly, calculate the amount of oil obtained, the amount of oil obtained per 1 gof plant seed and the percent extraction efficiency by establishing la mass conservation balance around the rotary evaporator. Weighing result (g) The tare of the sampled balloon 35 Initial mass (balloon + solvent + oil) 88.13 Tare the balloon in which the solvent was collected 45 93.47 At the end of the experiment, the balloon + solvent mass in which the solvent is collectedarrow_forward
- You have to use systems of equations to solve the problem. Write down the systems you are using on paper and then use Matlab to solve the systems. Interprete your results from the Matlab computations again on your paper and give the final answers on the answer sheet. Balance the following chemical equation: _PbCrO4+ -- HCl + ----FESO4 → ----P6C12 + ----Cr2(SO4)3 + ----FeCl3 + ---H2O+ ----Fe2(SO4)3arrow_forward7arrow_forward2. In an ethanol production plant, a separator produces a 99% ethanol product from a feedstock stream containing 85% water and 15% ethanol at a rate of 450 Ib/min. The separator has two outlet streams: the ethanol product outlet stream (99% ethanol) and a residual water stream. 30% of the feedstock is bypassed and mixed with the residual water stream leaving the separator. If 60% of the ethanol entering the separator is recovered in the product stream, determine the composition of the residual water stream just after leaving the separator and the composition of the residual stream after mixing with the bypass stream.arrow_forward
- (1) - denaitar... Sign In - Oasis Discover Student L. publix passport Canvas A ALEKS - Denait A O CHEMICAL REACTIONS Predicting the products of a neutralization reaction Predict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is balanced. HC10, + NaOH 1 Explanation Check O 2021 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Center Type here to search 58°F 近arrow_forwardA standard reference material is certified to contain 94.6 ppm of an organic contaminant. Your analysis gives you the follow values, 98.6, 98.4, 95.1 and 97.2 ppm. Can you say with 95% confidence that your values differ from the certified value. Please show workarrow_forwardWe want to inform you that your performance as a tutor is being evaluated and will be reported to the administration. Additionally, there will be an online ranking system that not only assesses the overall performance of Bartleby as a website but also ranks individual tutors based on their question-and-answer accurate contributions. This ranking will be shared with management. Please ensure that your responses are of the highest quality, as multiple requests may be directed to the same person for assessment.arrow_forward
- www-awn.aleks.com ALEKS - Brittney Ortega - Learn Answered: A major component of gasoline is octane... | bartleby CHEMICAL REACTIONS Brittney V Solving for a reactant using a chemical equation Green plants use light from the Sun to drive photosynthesis. Photosynthesis is a chemical reaction in which water (H,O) and carbon dioxide (CO,) chemically react to form the simple sugar glucose (CH,1206) and oxygen gas (02). What mass of oxygen gas is produced by the reaction of 3.7 g of water? Round your answer to 2 significant digits. alo g Ar х10 Explanation Check © 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy | Accessibilityarrow_forwardMBA Corp plans to use its idle building that can potentially be rented for $15,000 per annum to set up a manufacturing machinery there. The current Revenue of MBA Corp is $500,000. If the company takes up this project, its revenue is expected to increase by 30% for the next three years and then double (from the 3rd year level) for the next two years. After 5 years, the project will be scrapped and the salvage value is expected to be $80,000 The COGS are expected to remain the same at 60% of revenue. The SG&A and other operating costs will increase by $10,000. The cost of the machinery is expected to be $250,000. The machinery installation cost is expected to be $20,000. This investment will require additional inventory of $40,000 and increase the accounts payable by $20,000 The company spent $ 5000 in researching the viability of the building for machine installation. The company hires you as a financial manager to advise if they should take up this project or not. Other information:…arrow_forwardThe three major steps in the QA process are Use objectives, fortification, blanks Use objectives, specifications, assessment Use objectives, false positives, raw data Fortification, blanks, calibration testing Raw data, treated data, resultsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY