
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
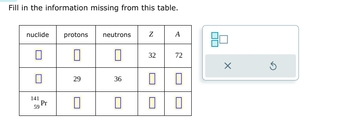
Transcribed Image Text:Fill in the information missing from this table.
Ꮓ
A
☐
32
72
nuclide protons neutrons
☐
141
59
Pr
29
36
☐
☐
☐
☐
☐
☐
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 5 images

Knowledge Booster
Similar questions
- Atoms are electrically neutral. This means that an atom will contain a.more protons than neutrons. b.more electrons than protons. c.an equal number of protons and electrons. d.None of the above.arrow_forward2-85 The mass of a proton is 1.67 × 10-24g. The mass of a grain of salt is 1.0 × 10-2g. How many protons would it take to have the same mass as a grain of salt?arrow_forwardOxygen consists of three different _____, each having eight protons but different numbers of neutrons.arrow_forward
- Knowing the number of protons in the atom of a neutral element enables you to determine which of the following? the number of neutrons in the atom of the neutral element the number of electrons in the atom of the neutral element the name of the element two of the above none of the above Explain.arrow_forwardFill in the following table:arrow_forwardWhich of the following is{are) correct? a. 40Ca2 contains 20 protons and 18 electrons. b. Rutherford created the cathode-ray tube and was the founder of the charge-to-mass ratio of an electron. c. An electron is heavier than a proton. d. The nucleus contains protons, neutrons, and electrons.arrow_forward
- The following table gives the number of protons and neutrons in the nuclei of various atoms. Which atom is the isotope of atom A? Which atom has the same mass number as atom A? Protons Neutrons Atom A 32 39 Atom B 33 38 Atom C 38 50 Atom D 32 38arrow_forwardThe formula of water is If-O. Which of the following is indicated by this formula? Explain your answer. a. The mass of hydrogen is twice that of oxygen in each molecule. b. There are two hydrogen atoms and one oxygen atom per water molecule. c. The mass of oxygen is twice that of hydrogen in each molecule. d. There are two oxygen atoms and one hydrogen atom per water molecule.arrow_forwardMatch these by placing the correct notation in the appropriate blank. 3467Se3367As3567Br3672Kr a. Contains 33 neutrons b. Contains greatest number of neutrons c. Contains equal number of protons and neutrons d. Contains the same number of neutrons as there are protons in As-67arrow_forward
- The following isotopes have applications in medicine. Write their symbols in the form XZA. a. cobalt-60 b. phosphorus-32 c. iodine-131 d. sulfur-35arrow_forwarda. Classify the following elements as metals or nonmetals: Mg Si Rn Ti Ge Rn Au B Am Bi At Br b. The distinction between metals and nonmetals is really not a clear one. Some elements, called metalloids, are intermediate in their properties. Which of these elements would you reclassify as metalloids? What other elements in the periodic table would you expect to be metalloids?arrow_forwardYou perform a chemical reaction using the hypothetical elements A and B. These elements are represented by their molecular models shown below: The product of the reaction represented by molecular models is a Using the molecular models and the boxes, present a balanced chemical equation for the reaction of elements A and B. b Using the symbols A and B2 for the chemical reaction, write a balanced chemical equation. c What are some real-element possibilities for element B?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning