
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please correct answer and don't use hand rating
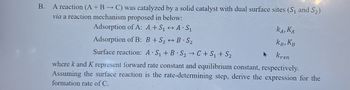
Transcribed Image Text:B.
A reaction (A + B → C) was catalyzed by a solid catalyst with dual surface sites (S1 and S2)
via a reaction mechanism proposed in below:
Adsorption of A: A+S₁
A S₁
Adsorption of B: B+S2B S₂
Surface reaction: A S₁+B S2C+S₁ + S₂
KA, KA
KB, KB
A
Krxn
where k and K représent forward rate constant and equilibrium constant, respectively.
Assuming the surface reaction is the rate-determining step, derive the expression for the
formation rate of C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- The acid-catalyzed iodination of acetone CH3COCH3(aq) + I2(aq) CH3COCH2I(aq) + HI(aq) is a common laboratory experiment used in general chemistry courses to teach the method of initial rates. The reaction is followed spectrophotometrically by the disappearance of the color of iodine in the solution. The following data (J. P. Birk and D. L Walters, Journal of Chemical Education, Vol. 69, p. 585, 1992) were collected at 23 C for this reaction. Determine the rate law for this reaction.arrow_forwardThe oxidation of iodide ion by the hypochlorite ion in the presence of hydroxide ions I(aq) + ClO(aq) IO(aq) + Cl(aq) was studied at 25 C, and the following initial rates data (Y. Chia and R. E. Connick, Journal of Physical Chemistry, Vol. 63, p. 1518, 1959) were collected: (a) Determine the rate law for this reaction. (b) One mechanism that has been proposed for this reaction is the following: Show that the rate law predicted by this mechanism matches the experimentally determined rate law in part a. (Note that when writing the expression for K the equilibrium constant, [H2O] is not involved. See Chapter 15.)arrow_forwardThe color change accompanying the reaction of phenolphthalein with strong base is illustrated below. The change in concentration of the dye can be followed by spectrophotometry (Section 4.9), and some data collected by that approach are given below. The initial concentrations were [phenolphthalein] = 0.0050 mol/L and [OH] = 0.61 mol/L. (Data are taken from review materials for kinetics at chemed.chem.purdue.edu.) (For more details on this reaction see L Nicholson, Journal of Chemical Education, Vol. 66, p. 725, 1989.) (a) Plot the data above as [phenolphthalein] versus time, and determine the average rate from t = 0 to t = 15 seconds and from t = 100 seconds to t = 125 seconds. Does the rate change? If so, why? (b) Use a graphical method to determine the order of the reaction with respect to phenolphthalein. Write the rate law, and determine the rate constant. (c) What is the half-life for the reaction?arrow_forward
- Instantaneous rates for the reaction of hydroxide ion with Cv+ can be determined from the slope of the curve in Figure 11.3 at various concentrations. They are (1) At 4.0 105 mol/L, rate = 12.3 107 mol L1 s1 (2) At 3.0 105 mol/L, rate = 9.25 107 mol L1 s1 (3) At 2.0 105 mol/L, rate = 6.16 107 mol L1 s1 (4) At 1.5 105 mol/L, rate = 4.60 107 mol L1 s1 (5) At 1.0 105 mol/L, rate = 3.09 107 mol L1 s1 (a) What is the relationship between the rates in (1) and (3)? Between (2) and (4)? Between (3) and (5)? (b) What is the relationship between the concentrations in each of these cases? (c) Is the rate of the reaction proportional to the concentration of Cv+? Explain your answer.arrow_forwardThe following experimental data were obtained for the reaction of \'I14* and NOf in acidic solution. NH/(aq) + NO2-(aq) — N;(g) + 2 H,O(f) INH/I (mol L1) [NO21 (mol L-1, Rate = A[NJ/At (mol L-1 s’) 0.0092 0.098 3.33 X IO"7 0.0092 0.049 1.66 X 10‘7 0.0488 0.196 3.51 X 10"6 0.0249 0.196 1.80 X 10-6 Determine the rate law for this reaction and calculate the rate constant.arrow_forwardFor the past 10 years, the unsaturated hydrocarbon 1, 3-butadiene (CH2 = CH - CH = CH2) has ranked 38th among the top 50 industrial Chemicals. It is used primarily for the manufacture of synthetic rubber. An isomer exists also as cyclobutene: The isomerization of cyclobutene to butadiene is first-order and the rate constant has been measured as 2.0104s1 at 150 C in a 0.53-L ?ask. Determine the partial pressure of cyclobutene and its concentration after 30.0 minutes if an isomerization reaction is carried out at 150 C with an initial pressure of 55 torr.arrow_forward
- Answer following parts only. a(ii) , carrow_forwardPLEASE ANSWER BOTH i) and ii) !!!!arrow_forwardNH3 is used as an agent for NO reduction in the temperature range of about 1,000 K. The mechanism for NO reduction by NH; contains the following reaction steps: NH3 + OH NH2 + H2O (RI) NH2 + NO – N2 + H+ OH (R2) NH, + NO – N, + H,O (R3) H+02 -0+ OH (R4) O+ H2O → OH+ OH (R5) Show that the above process runs away when the (branching) ratio of the rate of the branching reaction (R2) over that of the termination reaction (R3) exceeds the critical value of 1/3. Assume that NH2, H, and O are in steady state.arrow_forward
- (1) The gas phase decomposition of dinitrogen pentoxide at 335 KN2O5(g)2 NO2(g) + ½ O2(g)is first order in N2O5 with a rate constant of 4.70×10-3 s-1.If the initial concentration of N2O5 is 3.97×10-2 M, the concentration of N2O5 will be 8.22×10-3 M after ______s have passed. (1b) The gas phase decomposition of sulfuryl chloride at 600 K SO2Cl2(g) SO2(g) + Cl2(g) is first order in SO2Cl2 with a rate constant of 2.80×10-3 min-1. If the initial concentration of SO2Cl2 is 4.90×10-3 M, the concentration of SO2Cl2 will be M after ______ 499 min have passed.arrow_forwardE17D.4(b) The activation energy for the decomposition ofbenzene diazonium chloride is 99.1 kJ mol". At what temperature is the rate constant 10 per cent greater than at 25 °C? 16arrow_forwardGivena reaction 2A + B---> C + 2D which is found to be second order in a and independent of B, is the proposed slow step of the mechanism 2A--> D + E consistent with the experimental data? I don't think so, but i would like confirmation.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning