
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Courses
Question 15 - Electrochemistry la X
b My Questions | bartleby
X * physical chemistry - Positive or N X +
ezto.mheducation.com/ext/map/index.html?_con=con&external_browser=0&launchUrl=https%253A%252F%252Fnewconnect.mheducation.com%252F#/activity/q...
2
Аpps
My STC
Mail - Janeth Soto -...
CengageNOW | On..
Hidalgo County
Yahoo Mail
M HBO Max
ScheduleConnect
Colonia Self-Help C..
E Reading list
Electrochemistry lab alternative i
Help
Save & Exit
Submit
Saved
15
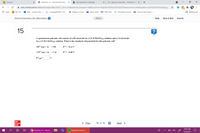
A spontaneous galvanic cell consists of a Pb electrode in a 1.0 M Pb(NO3)2 solution and a Cd electrode
in a 1.0 M Cd(NO3), solution. What is the standard cell potential for this galvanic cell?
2+
Pb+* (aq) + 2е — РЬ
Е 3 -0.13 V
Cd2*(ag) + 2e→ Cd
Е 3 —0.40 V
E°.
V
%3D
cell
Mc
Graw
Hill
< Prev
15 of 18
Next >
8:39 AM
Question 15 - Electr...
Spotify Premium
5/3/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Mon Apr Content X b Answered: How many mL of X Bb SolutionsQuiz(2) D(175) Destiny's Child S x + thos.content.blackboardcdn.com/5ebc429344443/2748179?X-Blackboard-Expiration=D1619492400000&X-Blackb... Q ☆ be C Home | Chegg.com My Library Brytew... 6 Introductory Che.. 2 / 2 126% + | A How many mL of a 4 M Al(OH)3 solution are needed to reaction with 125 mL of a 2 M HCl solution? 6. Al(OH)3 (s) + 3 HCl (aq) З НСІ → AICI3 (aq) + 3 H2O (1)arrow_forwardCH,(CH,),CHO $ Incorrect 27 F R V % G Search or type URL 5 T B tv 6 Y MacBook Pro H 9 N & 7 U J t. OH * 8 M I K ( 9 < 36 O V A Ⓒ ) O L P { ? I Tarrow_forwardEmerson M Cremistr pdated late workE a educate.lindsay.k12.ca.us/iFrame.aspx?iCtri=STUDENT BASE HOME CONTROL O Empower 2021 O Ermpower 2021 pokmarks DE IXL Classes O Google Slides O Empower 2020 A Google Docs t Lindsay High School Physics Safety & Ski. E Guided pract 2-1 googlegalaxysclence.com Li Be 1-0 1-5 2-0 Al 2.5 3-0 3-5 Na Mg 1-2 Si S CI 0-9 1-5 1-8 Ge 2-1 2-5 3-0 K Ca Ga 1-6 As Se Br 0-8 1-0 1-8 2-0 2-4 Te 2-8 Rb Sr In Sn Sb 0-8 1-0 1-7 1-8 Pb 1-9 2-1 2-5 Cs 0-7 Ba TI Bi Po At 2-2 Review the chart above. It is showing how much energy it takes to remove an electron from each of those types of atoms. Why would 0.9 1-8 1-9 1-9 2-0 it be so high in the top right comer and so low in the lower len comer? It would be high in the top right coner because these electrons are closer to the nucleus and held more strongly, so they will take O A. more energy to remove. And the opposite is true for the lower left, farther from the nucleus and so not held as strongly, requiring less energy to…arrow_forward
- ← Chrome File Edit View History Bookmarks Profiles Tab Evolution of the Gex tab → 13.1 Solution Form.... M Gmail YouTube Maps Ghess law organic c..... M esc caps lock 75% D Sat 10:20 PM a Gp and reactants-G X Significant Figures X Bb Department of Che X M Mathway | Algebra x A ALEKS-Lara Althax www-awu.aleks.com/alekscgi/x/isl.exe/1o_u-IgNsikr7j8P3jH-lvTqeviKFP6W0cqJcWJdIACROQwyw24GWHin4XFyQvsTuo6422Ls2TWa4xMOJZaDSVCHQol7p3... ☆ O KINETICS AND EQUILIBRIUM Using Le Chatelier's Principle to predict the result of changing... Carbon disulfide and oxygen react to form carbon dioxide and sulfur dioxide, like this: CS₂(g)+30₂(g) → CO₂(g) +2SO₂(g) perturbation Some O₂ is removed. Some CO₂ is added. Suppose a mixture of CS₂, O₂, CO₂ and SO₂ has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. Explanation 1…arrow_forwardn Home Work-2 (page 7 of 8) b My Questions | bartleby A moodle.nct.edu.om/mod/quiz/attempt.php?attempt=709737&cmid=76257&page=6 E Apps * Bookmarks تحويل كيلومتر إلى م. .. © E Reading list NCT e-Learning Portal Courses Reports - e-Services Academic Departments - ETC - CIMS - Muayid Mahmood Mohammed Al Azri Fundamentals Of Chemistry (Engineering) Dashboard / My courses / CHEM1100 / Home works / Home Work-2 Quiz navigation Question 7 How many Faraday is needed to deposit 1.8 g of Sodium (Na) from NaCl solution using electrolysis process. Not yet 1 2 3 4 5 6 8 answered Answer: Marked out of Finish attempt . 1.00 P Flag question Time left 191:45:56 Previous page Next page - Submission Link for Home Work-1 Jump to. Quiz 1 for Section-3 - You are logged in as Muayid Mahmood Mohammed Al Azri (Log out) CHEM1100 Data retention summary. Get the mobile app 10:13 PM P Type here to search 72°F Clear O E 1 G 4) ENG 12/14/2021arrow_forward100% Реople Tab Window Help File Edit View History Bookmarks Chrome Q Solutions and Interm x Q Chemistry- Solution x Answered: What is 101 Chem101 Q C16 Chemistry Ch16 x ... B Lecture Chapter 9 -> i app.101edu.co O YouTube M Gmall A Maps B #14 CRM Study B.. A (16) Piggy (ALPH. E Apps American Express.. ud Urban Dictionary:. O News Translate Question 16 of 35 Convert the concentration of 0.700 M Na,so, to g/mL X STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 83.3 142.04 0.0994 6.022 x 1023 1000 1 119.05 99.4 0.700 0.001 0.0833 mL M Na,SO, g Na* mol Na* g/mL mol NazSO, g Nazso, 回■O區民@T山 MacBook Pro #3 1 3 4. & 6. 7. 8. Q W Tarrow_forward
- Help 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forwardI need help with part D pleasearrow_forward°F rtly cloudy m/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-lvWyv8WYLP6W0cqJcWJdIACROQwyw24GWHInMM72ts 1 NTBcSzwZOGGhrmIP4yCejeRhX9HIzqlzRTj2iaDBXTCRIHYWNO_-o?10... O CHEMICAL REACTIONS Calculating molarity using solute moles A chemist prepares a solution of mercury(II) iodide (HgI₂) by measuring out 0.0161 μmol of mercury(II) iodide into a 300. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in μmol/L of the chemist's mercury(II) iodide solution. Round your answer to 3 significant digits. µ mol L Explanation Check x10 X Ś Q Search 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use **************** D 1/5 www Jessi Privacy Center | Accessibilityarrow_forward
- I don't understand How to do this question and I am on my last try please help me!arrow_forwardFile Edit View History Bookmarks Profiles Tab Window Help 7 YouTube Maps = tab liquid x N. Ask Yox N. Ask Yo x A ALEKS X www-awu.aleks.com/alekscgi/x/1sl.exe/1o_u-IgNslkr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHintXeoleXL2H OSTATES OF MATTER Using heat of fusion or vaporization to find the heat needed to... esc Explanation 39 cto X 1 Q A 2 Gheat ox v Calculate the amount of heat needed to melt 88.1 g of solid methanol (CH3OH) and bring it to a temperature of -23.6 °C. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol. 0 Z Check W S 3 X g X E D $ 4 C S > R F % 5 I tv T V 6 G B Thank Y 7 H U G 111 ck X N The 8 © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 274@ A Aa O J Sketch X New 1 x + GCwaDdYUtqAJT1yasCs1fG_rUoyaRSVpsjfsOdH... ☆ 0 1 M 9 ( K O < 431% D ge Gwet dr x 0 L Wed 9:43 PM 0/5 P command Lara V ? 1 dlo Aarrow_forwardFile Edit View History Bookmarks Profiles Tab Window Help e Watch Gilmore Girls X * Dementia Friend Ce X y Yahoo Lobby Top Ha A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNsIkr7j8P3jH-IVDWKW_BBZZ16tTytly4Fcfu6: Spotify Web Playe... M Common Ethical D... O THERMOCHEMISTRY Calculating kinetic energy km Calculate the kinetic energy of a 3.2 x 10° kg satellite moving at a speed of 3.2 Round your answer to 2 significant digits. Elanation Check 13 IIarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY