
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:activity - Saved to itslearning
P Search
Layout
References
Review
View
Help
O Editing v
s New Ro.. v 12
A
B
I
U
Ev Ev E E E A-
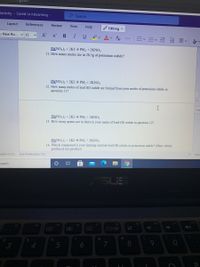
Pb(NO3)2 + 2KI → Pbl, + 2KNO3
11. How many moles are in 28.5g of potassium iodide?
Pb(NO3)2 + 2KI → PbI2 + 2KNO3
12. How many moles of lead (II) iodide are formed from your moles of potassium iodide in
question 11?
2
I
Hea
Pb(NO3)2 + 2KI → PbI, + 2KNO3
13. How many grams are in there in your moles of lead (II) iodide in question 12?
Pb(NO3)2 + 2KI → PbI2 + 2KNO3
14. Which compound is your limiting reactant lead (II) nitrate or potassium iodide? (Hint: which
produced less product)
nglish (U.S.)
Text Predictions: On
90%
earch
SUE
f8
f9
F10
14
(5
3
4
5
7
8
9.
Transcribed Image Text:Hing.com
Get?Fileld-hNDLmdusbEmplc8ue%2laZn4otcFEREdCkEOBEgn%2bHNOn13v5HvafUMjlu77SxrKT
vity - Saved to itslearning
P Search
Layout
References
Review
View
Help
O Editing v
w Ro... v 12
A^
U
ev Av A
=<而< 向 川<
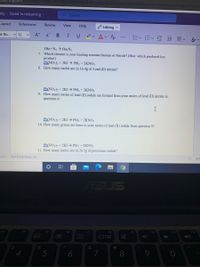
3Ba+ N2 → BazN2
7. Which element is your limiting reactant Barium or Nitride? (Hint: which produced less
product)
Pb(NO3)2 + 2KI → PbI, + 2KNO3
8. How many moles are in 16.4g of Lead (II) nitrate?
Pb(NO3)2 + 2KI → PbI2 + 2KNO3
9. How many moles of lead (II) iodide are formed from your moles of lead (II) nitrate in
question 8?
Pb(NO3)2 + 2KI → PbI, + 2KNO3
10. How many grams are there in your moles of lead (II) iodide from question 9?
Pb(NO3)2 + 2KI → PbI2 + 2KNO3
11. How many moles are in 28.5g of potassium iodide?
909
U.S.)
Text Predictions: On
ASUS
f10
(12
f5
f6
f8
f9
&
6
7
8
因
Expert Solution
arrow_forward
Step 1
Since you have posted questions with multiple sub-parts, we are entitled to answer the first 3 only.
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In each chemical reaction below, an atom or group of atoms is moving. It is bonded to something different in the reactants than it is in the products. A chemical reaction always involves a move like this. 18. A. B. C. D. 19. PAAUA ARCA D. 20. A. B. C. 9.1 0Z (258g) D. 4 H2 + CO2 → CH4 + 2 H₂O mie Which atoms is the C bonded to in the reactants? (C) Which atoms is the C bonded to in the products? en (H) Which atom is each O bonded to in the reactants? (0) Which atoms is each O bonded to in the products? (C) HCl + NaOH → NaCl + HOH Which atom is the Cl bonded to in the reactants? Which atom is the Cl bonded to in the products? Which atom is the OH group bonded to in the reactants? Which atom is the OH group bonded to in the products? will pieces to leave all CH4 + 2 O2 CO₂ + 2 H₂Oith the labi, please Which atom is the C bonded to in the reactants? Which atom is the C bonded to in the products? Which atom is the O bonded to in the reactants? Which atom is the O bonded to in the products?…arrow_forwardI need an answer for this question.arrow_forwardHow many sodium ions are present in 20.0 mL of 0.10 M Na2Sarrow_forward
- Carbonic acid can form water and carbon dioxide upon heating. How much carbon dioxide is formed from 6.20 g of carbonic acid? H₂CO3 → H₂O + CO₂ 2.20 g 8.80 g 6.20 g 4.40 garrow_forward: ☐ + T ☑ Add the necessary reaction conditions above or below the arrow in this organic reaction. Also, if a major product is missing from the right-hand side, draw it in. m ↑ .OH - ' ||||||arrow_forward41. Potassium permanganate, KMnO4, a common oxidizing agent, is made from various ores that contain manganese (IV) oxide, MnO2. The following equation shows the net reaction for one process that forms potassium permanganate. 2MnO2 + 2KOH + O2 → 2KMnO4 + H2 a. What is the maximum mass, in kilograms, of KMnO4 that can be made. from the reaction of 835.6 g of MnO2 with 585 g of KOH and excess oxygen gas? b. Explain why the oxygen gas is in excess. c. If 1.18 kg of KMnO4 are isolated from the product mixture of the reaction of 835.6 g of MnO2 with 585 g of KOH and excess oxygen gas, what is the percent yield?arrow_forward
- MISSED THIS? Watch KCV 8.2: Read Section 8.3. You can click on the Review link to access the section in your eText. For the reaction shown, calculate how many moles of NO₂ form when each amount of reactant completely reacts. 2 N₂Os (g) → 4NO₂2 (9) + 0₂ (9) Y Part A 1.7 mol N₂O5 Express your answer using two significant figures. 15. ΑΣΦΑ Submit Part B Submit Request Answer 6.1 mol N₂O5 Express your answer using two significant figures. 5 ΑΣΦ Part C Request Answer ? ? mol molarrow_forwardLithium reacts with thionyl chloride to form lithium chloride, sulfur, and sulfur dioxide. 4 Li + 2 SOCI2 - 4 LİCI + S + SO2 Determine the mass of SO2 (molar mass = 64.07) formed when 5.5 g SOCI2 (molar mass = 118.97) reacts with excess Li. 2.8 g O 1.5 g 0.36 g O 8.4 g O 4.2 garrow_forwardPlease don't provide hand writtin solution....arrow_forward
- Just give me the last step solutions(make the solution shortest) and give me the answer. Thank you.arrow_forward18. Choose the correct answer and show the step by step solution.arrow_forward40 Magnesium reacts with sulfuric acid according to the fol- lowing equation. How many moles of H, are produced by the complete reaction of 230. mg of Mg with sulfuric acid? 2. Mg(s) + H,SO¸(aq) → MgSO,(aq) + H,(g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY