
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:nrome
File Edit View History Bookmarks People Tab Window Help
101 Chem101
* 9%D Sat 1:09 PM
Q O E
O General Chemistry I (LAB SC x +
i app.101edu.co
10 Less Known Sleep Dis.
O www.youtube.com
Recommended: Psych20o
Close
os M Gmail O
O Maps A Translate
U https://www.carth.
Settings
Google Chrome isn't your default browser
Set as default
Question 6 of 7
Submit
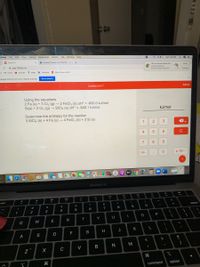
Using the equations
2 Fe (s) + 3 Cl2 (g) → 2 FeCl3 (s) AH° = -800.0 kJ/mol
Si(s) + 2 Cl2 (g) → SiCla (s) AH° = -640.1 kJ/mol
kJ/mol
Determine the enthalpy for the reaction
3 SiCl4 (s) + 4 Fe (s) → 4 FeCl3 (s) + 3 Si (s)
3
4
C
8
9.
+/-
x 100
1122
10,068
OCT
étv
24
MacBook Air
80
888
FS
esc
F2
FA
F3
&
$
delete
7
8
1
2
P
Q
W
E
R
Y
J
K
A
S
D
F
G
>
?
N
M
C
V
command
option
.. *-
-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- K Haylee Scott - cp Zoom meeting lin com/web/viewer.html?state%=D%7B'ids %3A%5B"1zOflgo1GoQFrdviJ6PWx8L3h2j4uav5n"%5D%2C"action"%3A'open C Clever | Portal A Classroom https://sso.theleam. M Oro Grande Elemen. W Yearbook Aven ue Period 5 Scien. Haylee Scott - cprr061.pdf Building Vocabulary From the list below, choose the term that best completes each sentence. matter physical change endothermic reaction chemical change chemistry precipitate exothermic reaction 7. Any change that alters a substance without changing it into another substance is a(n) 8. is anything that has mass and takes up space. 9. A reaction that releases energy in the form of heat is called a(n) 10. A(n) absorbed. 11. A chemical reaction is also referred to as a(n) is a reaction in which energy is 12. A(n) a chemical reaction. is a solid formed from a solution during 13. is the study of the properties of matter and how matter changes. OPearson Education, Inc., publishing as Pearson Prentice Hall. All rights reserved…arrow_forward45- 40 35- 30- 25 20 10 Amount of Water Added (milliliters) Average Reaction Time (seconds)arrow_forwardi Chrome File Edit View History Bookmarks People Tab Window Help Chem101 O General Chemistry I (LAB SC x + * 8%D Sat 1:10 PM Q OE i app. 101edu.co 10 Less Known Sleep Dis www.youtube.com Recommended Psych2Go Close E Apps M M Gmail D YouTube Maps Translate 3 https://www.carth. Settings O Google Chrome isn't your default browser Set as default Question 8 of 8 Submit A 130.3 g piece of copper (specific heat 0.38 Jİ •°C) is heated and then placed into 400.0 g of water initially at 20.7°C. The water increases in temperature to 22.2°C. What is the initial temperature of °C the copper? (The specific heat of water is 4.18 J/g.°C). 1 3 4 6 C 8 9 +/- х 100 10,058 dtv 24 MacBook Air 80 esc $ & %23 delete 4 6 7 8 9. 1 3 P Q W E R т Y F G J K retur A D s lock > V N M z' H command option option command control R 332 2.arrow_forward
- n Home Work-2 (page 7 of 8) b My Questions | bartleby A moodle.nct.edu.om/mod/quiz/attempt.php?attempt=709737&cmid=76257&page=6 E Apps * Bookmarks تحويل كيلومتر إلى م. .. © E Reading list NCT e-Learning Portal Courses Reports - e-Services Academic Departments - ETC - CIMS - Muayid Mahmood Mohammed Al Azri Fundamentals Of Chemistry (Engineering) Dashboard / My courses / CHEM1100 / Home works / Home Work-2 Quiz navigation Question 7 How many Faraday is needed to deposit 1.8 g of Sodium (Na) from NaCl solution using electrolysis process. Not yet 1 2 3 4 5 6 8 answered Answer: Marked out of Finish attempt . 1.00 P Flag question Time left 191:45:56 Previous page Next page - Submission Link for Home Work-1 Jump to. Quiz 1 for Section-3 - You are logged in as Muayid Mahmood Mohammed Al Azri (Log out) CHEM1100 Data retention summary. Get the mobile app 10:13 PM P Type here to search 72°F Clear O E 1 G 4) ENG 12/14/2021arrow_forwardA McGraw-Hill Connect O Question 5 - Homework 3 - Co X Q CHE 120 Chapter 1 Flashcards x + X A ezto.mheducation.com/ext/map/index.html?_con=con&external_browser=0&lau.. O 用 Apps Netflix h Hulu O Grammar Checker A Canvas Prime Video A Other Bookmarks 国Readi >> Homework 3 O Save & Exit Saved Help Submit 2 attempts left Check my work Enter your answer in the provided box. 1 points Calculate the mass fraction of Ca in calcium bromate. mass fraction Ca eBook Print References Mc Graw Hillarrow_forwardNeed complete solutions no need ai solutions okk just solve accuratearrow_forward
- Egwuekwe-Ou x * Office Editing for Docs, Sheets & Slides| chrome-extension://bpmcpldpdmajfigpchkicefoigmkfalc/views/app.html aboutblank ALL2022 (1).doc Format Tools Help ~ HQ Q Normal Unsaved change 12 M HCI should we use? a. 3 L b. 1 L c. 2 L Edits will not be automatically saved. Times New ... e 28. 4 M solution of NaOH means a. 4 moles of NaOH in 1 L b. 4 grams of NaOH in 1L c. 4 moles of NaOH in 100 mls about:blank 31. What is a catalyst? 27. We have 12 M HCl and need 2 L of 6 M HCl. How many Liters of 30. Equilibrium is the state in which 12 ▾ X W FINALFALL2022 (1).doc Save now BIU A A 29. A gas mixture of N₂, O₂ and He exerts a total pressure of 1 atmosphere. N₂ exerts a pressure of 0.2 atmospheres. O₂ exerts a pressure of 0.5 atmospheres. What is the pressure of He? a. The rate of the forward reaction is greater than the reverse reaction. b. The rate of the reverse reaction is greater than the forward reaction. c. The rate of the forward reaction and reverse reaction are equal.…arrow_forwardCom X Bb Mas X Mas X Hom X K! Kahx Exan X K! Kah X Mitc X КI Kah X K Kah x K! Kah x Kah x K Kahx C session.masteringchemistry.com/myct/itemView?assignmentProblemID=134214560 Apps New Tab Effect of Salinity on... Sleep Disorders | M... P Philo American Journal ... Zeeshan JBAS Ic Wait for Next Quest... oramge juice Effect carrow_forwardHi I need help with this questions pleasearrow_forwardE MasteringChemistry: HW 01b C Get Homework Help With Che b My Questions | bartleby > MyLab and Mastering Home - Students x X A session.masteringchemistry.com/myct/itemView?offset=next&attemptNo=1&assignmentProblemlD=139293464 E Apps W California Fool's Go... CLA Purdue OWL: APA F.. O Christmas Window... C Bianca 4-piece Fabr.. O ANGELINO o How do I format m. O Choosing Resource. New Tab Dashboard >>arrow_forwardhrome Edit View History Bookmarks Profiles Tab Window Help File Watch Gilmore Girl: x * Dementia Friend CX y Yahoo Lobby | Top Hat A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IvdWKW BBZZ16tTytly4Fcfu6zOtOf8c Spotify Web Playe... M Common Ethical D.. O THERMOCHEMISTRY Calculating kinetic energy m Calculate the kinetic energy of a 569. kg roller-coaster car moving at a speed of 26.0 Round your answer to 3 significant digits. Explanation Check IIarrow_forwardInbo Ansv (534 Con I Balar Post CHE 101 C X The bartl bartl bartl Cher Unkr O Sear E I ma G what app.101edu.co/# Bryant's Gmail Cascadia Canvas Lo... T GSBA Scholarship L... HOMEGROWN TRA... Learn Touch Typing... C The Science of Well... Investor360° ® Login ClickUp Reading list >> Question 21 of 40 Submit KOH is a Brønsted-Lowry base because A) it is a polar molecule. B) it can dissolve in water. C) it is a hydroxide donor. D) it is a proton acceptor. 11:34 PM e Type here to search 59°F 小 8/26/2021 (8)arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_iosRecommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY