
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Cyclohexane (C6H12) can be made by the reaction of benzene (C6H6) with hydrogen according to the
following reaction:
C6H6 + H2 → C6H12
For the process shown in the figure, determine the a) composition of the product and b) the ratio of
the recycle stream to the fresh feed. The overall conversion of benzene is 95% and the single pass
conversion is 20%. Assume that 20% excess hydrogen is used in the fresh feed, and that the
composition of the recycle stream is 22.74 mol% benzene and 78.26 mole % hydrogen.
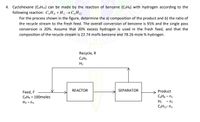
Transcribed Image Text:4. Cyclohexane (C6H12) can be made by the reaction of benzene (CsHs) with hydrogen according to the
following reaction: C,H, + H2 →C,H12
For the process shown in the figure, determine the a) composition of the product and b) the ratio of
the recycle stream to the fresh feed. The overall conversion of benzene is 95% and the single pass
conversion is 20%. Assume that 20% excess hydrogen is used in the fresh feed, and that the
composition of the recycle stream is 22.74 mol% benzene and 78.26 mole % hydrogen.
Recycle, R
C6H6
H2
REACTOR
SEPARATOR
Product
Feed, F
C6H6 = 100moles
CHs - n.
H2 -n2
CH12- n3
H2 - n4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- #3 The first step in the reaction sequence for the production of nitric acid via the oxidation of ammonia is: 4NH3 + 502 2 4NO + 6H2O 75% conversion is achieved with an equimolar mixture of ammonia and oxygen fed at the rate of 100 mol/h. Determine the outlet compositions. (Hint: determine the limiting reactant).arrow_forward3. Carbon tetrachloride (CC14) can be produced using the following reaction. If 34.535 grams of chlorine (Cl2) produced 28.550 g of CC14, what is the percent yield of this reaction? C(s) + 2 Cl2(g) CCI4(1)arrow_forwardA gaseous reaction, A→2B + C takes place isothermally in a constant pressure reactor. Staring with a gaseous mixture containing 50% A and (rest inert), the ratio of final to initial volume is found to be 1.6. The percentage conversion of A is...?arrow_forward
- check the image for questionarrow_forwardPlease answer the question with the steps... Thank youarrow_forward1. In a process for the manufacture of chlorine by direct oxidation of HCI with air over a catalyst to form Cl₂ and H₂O (only), the exit product is composed of HCI (4.4%), Cl₂ (19.8%), H₂O (19.8%), O₂ (4.0%), and N₂ (52.0%). The chemical reaction involved is as follow: 4HC1+02 → 2Cl2 + 2H₂0 Find a) limiting reactant and excess reactant, b) percent excess reactant, c) degree of completion of the reaction, d) extent of reactionarrow_forward
- A barytes composed of 100 percent BaSO4 is fused with carbon in the form of cokecontaining 6 percent ash (which is infusible). The composition of the fusion mass is:BaSO4 11.1%BaS 72.8%C 13.9%Ash 2.2 100% Reaction: BaSO4 + 4 C → BaS + 4 COFind the :4.1 Excess reactant. (10)4.2. The percentage of the excess reactant. (3)4.3. The degree of completion of the reaction. ?arrow_forward2. . Ethylene oxide (C₂H4O) is a high-volume chemical intermediate that is used in the manufacture of textiles, detergents, polyurethane foam, antifreeze, solvents, medicinal, adhesives, and other products like glycol and polyethylene glycol. Ethylene oxide is produced by the partial oxidation of ethylene (C₂H4) using a solid catalyst in a fixed-bed reactor: C₂H4 + O2 →→ C₂H4O An undesired reaction occurs where a portion of the ethylene reacts completely to form CO2 and H₂O: C₂H4 + O2- → CO₂ + H₂O The product gas leaving a fixed-bed ethylene oxide reactor has the following water-free composition: 22.5% C₂H4O; 70.7% N₂; 2.5% O₂; and 4.3% CO₂. Using molecular species balance, determine: a. the percent excess air based on the desired reaction b. the kg/h of the ethylene feed required to produce 75,000 MT/year of ethylene oxide c. the percent conversion of ethylene based on the desired reactionarrow_forwardFastarrow_forward
- Aniline is produced by the hydrogenation of nitrobenzene. A small amount of cyclohexylamine is produced as a by-product. Nitrobenzene is fed to the reactor as a vapor with three times the required stoichiometric amount of hydrogen. The conversion of nitrobenzene to the products is 96% and the selectivity to aniline is 85%. Unreacted hydrogen is separated from the reaction products and recycled to the reactor. From the recycle line it is purged to keep the inerts in the recycle stream below 5%. The fresh hydrogen that is fed is 99.5% pure and the rest is inert. Calculate the adiabatic outlet temperature of the reaction products and indicate the relationship with respect to the reference temperature (298.15) (in K):arrow_forwardW.W. Heckert et E. Mack studied the decomposition of ethylene oxide in gas phase at 25°C (C₂H4O → CH4 + CO), and found that it is a first-order reaction in the reactant. Determine the reaction rate constant using the date given below, for a reaction that begins with a pure reactant: time (min) Ptot (torr) 0 115.30 6 122.91 7 124.51 8 126.18 10 129.10arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The