
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Of these two methods, choose one that you would utilize for this compound for means of synthesizing. Explain why.
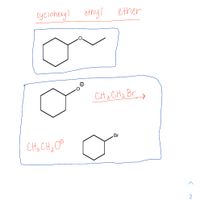
Transcribed Image Text:cyciohexyl
ethy!
ether
CH3 CH2 Br
.Br
CH3 CH, O°
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Deuterium (D) is an isotope of H. Both D and H have one proton and one electron; H has no neutrons and D has one. Consequently, D and H have nearly identical behavior, but they can be distinguished from each other experimentally due to their different masses. Therefore, replacing an H with a D in a molecule-deuterium isotope labeling-can provide valuable information about a mechanism. With this in mind, how would you synthesize each of the following deuterium-labeled compounds from the analogous unlabeled compound, using D,0 as your only source of deuterium? Hint: You will have to use two separate proton transfer reactions to synthesize each one. (a) (b) (c) OD D.arrow_forwardSpecify a synthetic scheme that would produce the compound shown above in the fewest steps possible. Use one of the starting materials shown together with any of the available reagents. Give the number of the starting material followed by the letters of the reagents in the order of their usearrow_forwardPlease help me with this question, it's urgent The reaction shown below does not follow the expected theory. Given what you learned about substitution/elimination, what is the expected mechanism this reaction should follow? Suggest why that is not what happens and give the actual product of the reaction.arrow_forward
- Write in line structuresarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the structure of the missing intermediates and products in the following mechanism. Include all lone pairs and charges as appropriate. Ignore stereochemistry. Ignore inorganic byproducts. (The structures there are incorrect and I do not know why. Please help. Thank you.)arrow_forwardShow ALL your steps, work and details in your calculations, use GRASPS: Given, Required, Analysis, Solution, and Paraphrase to answer the question, it is required. Answer in complete sentences and therefore statements.arrow_forward
- 2. Devise reactions schemes to carry out the following multi-stage processes to make ethyl ethanoate using ethanol as the only organic reagent а. b. to make 2- hydroxypropanonitrile from ethanol For each of the above show clearly the reagents you are using and the conditions and reagents needed for each stage of the conversions. You can illustrate your answer with equations or via mechanisms.arrow_forwardDescribe and explain the differences you would see when conducting propyl amine with dimethyl amine. Be specific on what you would make sure you could distinguish the two amines.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY