
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Consider a 17:0 fatty acid in a mammalian cell where propionyl CoA is completely oxidized.
For each fatty acid given, determine the following.
-
-
Gross ATP from b-oxidation cycles
-
Gross ATP from acetyl CoA produced
-
Gross ATP from conversion of propionyl CoA (if applicable)
-
Total number of ATP deducted
-
Total net ATP
-
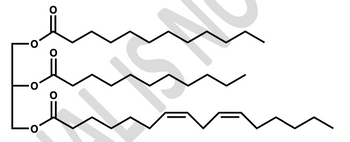
Transcribed Image Text:Lo
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Please explain and give the correct answerarrow_forwardConsider the reaction shown, which of the following conditions is most likely to lead to the activation of pyruvate dehydrogenase? high concentrations of acetyl-CoA high concentrations of NADH high concentrations of NAD+ high concentrations of CO2arrow_forwardif each NADH generates 3 ATP molecules and each FADH2 generates 2 ATP molecules, calculate the number of ATP molecules generated from one saturated 10-carbon fatty acid Determine the number of repetitions of the β‑oxidation spiral needed to completely degrade the fatty acid to acetyl-SCoAacetyl-SCoA (acetyl-CoA)(acetyl-CoA) . Calculate the ATPATP produced by the acetyl-SCoAacetyl-SCoA molecules in the citric acid cycle. Calculate the ATPATP produced from the FADH2FADH2 and NADHNADH produced from β‑oxidation. Add the ATPATP generated (from step 2 and 3), and subtract the ATPATP needed to activate the fatty acid.arrow_forward
- How many molecules of acetyl CoA, FADH2, and NADH are produced in the catabolism of a molecule of arachidonic acid, an 20-carbon carboxylic acid? How many times does the spiral reaction sequence occurarrow_forwardThe pyruvate dehydrogenase complex catalyzes the synthesis of acetyl-CoA, a very important molecule in cellular metabolism. The reaction catalyzed by the pyruvate dehydrogenase enzyme complex is shown below. This reaction is tightly regulated, because it is essentially irreversible. Which of the following conditions is most likely to lead to the inhibition of pyruvate dehydrogenase in cells? (select two answers) High concentrations of acetyl-CoA High concentrations of pyruvate High concentrations of NADH High concentrations of NAD+ High concentrations of CoAarrow_forwardUnder aerobic conditions when glucose is limiting, with high ratios of NADH/NAD+ and ATP/ADP, as carbon-2 radiolabeled pyruvate is utilized for its carbon skeleton, which molecules would you expect to see significant radiolabeling in the liver? Select all that apply. (multiple answers) Glucose C-2 only Label is halved over many TCA cycles Oxaloacetate Glucose C-1 and C-6 Glucose C-2 and C-5 CO2 from TCA cycle shows some radiolabel Lactate C-2 for export Malate Pyruvate C-1arrow_forward
- Under aerobic conditions of high ratios of NADH/NAD+ and ATP/ADP, as pyruvate is utilized for its carbon skeleton, which molecules would you expect to see significant radiolabeling in the liver? Select all that apply. **Please note some molecules contain more details, including not only molecule name, but location of the label. Pick the options that are accurate for the above situation. Glucose C-2 and C-5 Glucose C-1 and C-6 Glucose C-2 only Pyruvate C-1 Lactate C-2 for export CO, from TCA cycle shows some radiolabel Label is halved over many TCA cycles Oxaloacetate Malatearrow_forwardFatty acids are activated for breakdown through the action of acyl-CoA synthestase. Which of the following statements regarding acyl-CoA synthetase is not true? ● It catalyzes the addition of CoA to the fatty acid One of the products of the reaction is ADP. O The free energy change for the reaction catalyzed by this reaction is close to 0 kJ/mol, but the subsequent hydrolysis of pyrophosphate drives the reaction forward. O The reaction results in the formation of a thioester bond.arrow_forwardConsider a 24:1 △cis-9 fatty acid in the mitochondrion. For each fatty acid given, determine the following. 1. Gross ATP from b-oxidation cycles 2. Gross ATP from acetyl CoA produced 3. Gross ATP from conversion of propionyl CoA (if applicable) 4. Total number of ATP deducted 5. Total net ATParrow_forward
- Fill in the blanks below (input numbers only!) about the metabolism of hexanoic acid, a fatty acid that is one of the components of vanilla, and whose formula is CH3(CH2)4COOH: First, the fatty acid is activated by attaching CoA, which costs ATP molecules. The fatty acid is then broken down through a beta-oxidation spiral, to make acetyl CoA molecules. This will require "turns" of the beta-oxidation process. Since each turn of the cycle yields ATP molecules, and each acetyl COA will yield ATP molecules by going through the rest of its metabolism, the net ATP molecules produced from one molecule of this fatty acid will bearrow_forwardAcetyi CoA Oxaloncetate CoA NADH Citrate NAD Isocitrate Malate Funiarate NAD co NADH FADH, FAD a- Ketoghutarate Succinate co, NAD+ ATP Succinyl CuA NADH ADP - P For each molecule of glucose (C6H12O6) oxidized by cellular respiration, how many moles of CO2 are released in the citric acid cycle? (see the figure above) а. 2 b. 4 с. 6 d. 0 е. 3arrow_forwardIf oxidation of acetyl-CoA yields 10 ATPs per mole through the citric acidcycle, how many ATPs will be derived from the complete metabolic oxidation of 1 mole of alanine in a mammal? Would the corresponding energyyield in a fish be higher or lower? Why? How much energy would bederived from the metabolic oxidation of 1 mole of isoleucine to CO2, H2O,and NH3? Of tyrosine?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON