
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Transcribed Image Text:В
INITIAL
5.00
2.00
0.00
0.00
CHANGE
FINAL
4.00
0.00

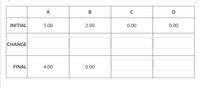
Transcribed Image Text:Complete the ICF (BCA) table below for the hypothetical chemical reaction. All values are in
moles. Enter all values with three digits (like shown) and include negative or positive signs for
the "Change" row.
A + 2B
ЗС + D
A
C
INITIAL
5.00
2.00
0.00
0.00
CHANGE
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CHAPTER 4-STOICHIOMETRY: QUANTITATIVE INFORMATION ABOUT CHEMIC Previous Page 3 of 4 Next → Iron ore is converted to iron metal in a reaction with carbon. If 4.00 mol of Fe2O3 is used, what amounts of Fe and CO2 are produced? Fill in the amounts table with your responses. Q Equation Initial (mol) Change (mol) Final (mol) 2 2 Fe₂O3(s) + 3 C(s) 11 W S 4.00 -4.00 0 tv 6.00 - 6.00 o E 0 S + 18 3 4 W AS R 4 Fe(s) Document3.docx. 0 % 6.5⁰ PACUPSTA PXnerienc IL.OMBI wenarchive T + MacBook Pro 6 Y & ★ 7 3 CO2 (9) 0 Yesterday at 6:04 AM AUO 75 70177 21 10.54 AM ★ U C Online t.... * 00 9arrow_forwardWhat is the conversion factor between two different substances in a chemical reaction? 81°F Mostly sunny Esc F1 1 {0 atomic numbers mole ratio molar mass F2 mass ratio -8- @ 2 F3 -Ő+ #M 3 FA 54 $ F5 % 5 F6 T Q F7 < 6 F8 & 7 F9 ★ * 00 8 F10 9 F11 ) F12 a P Prt Scarrow_forwardO Kf will change depending on what solute is dissolved in the solvent. QUESTION 11 Which term can be used to describe the process in the reaction below? 2 NaHCO3 (s) Na2CO3 (s) + H2O (g) + CO2 (g) Dissociation Precipitation Hydration Decomposition QUESTION 12 Which of the following would NOT be considered valid sources of error in a laboratory experiment? Click Save and Submit to save and submit. Cick Suve All Answers to save all ansicersarrow_forward
- I need help identifying which is the reactants, the product, the subscripts, the coefficient, and the yield sign (Not honor class) (Not grading)arrow_forwardcalculate how many moles of products would be produced if 0.500 mole of the first reactant were to react completely.arrow_forwardPlease help...the current balanced equation is incorrectarrow_forward
- A lab sample containing sodium chloride, ammonium chloride and sand. It initially weighs 3.207 grams, prior to heating. After heating that sample weighs 2.971 grams. What is the percentage of ammonium chloride in that initial mixture? Answer with the percentage, but do not include the percent sign.arrow_forwardElemental SS reacts with O2O2 to form SO3SO3 according to the reaction 2S+3O2→2SO3arrow_forwardComplete the following table, making sure to include your units! Concentration of Concentration of acetic acid Water level on measuring cylinder before reaction 98 mL 91 mL 95 mL 0.5% 0.2% 0.1% ● sodium bicarbonate 0.5 M 0.5 M 0.5 M From the results above, which reactant do you consider to be the limiting reagent? Why? ● Water level on measuring cylinder after reaction 8 mL 49 mL 81 mL Consider the reaction where the concentration of acetic acid was 0.5% and the concentration of sodium bicarbonate was 0.5 M. Complete the table on page 5 to calculate the theoretical yield of CO2 with these concentrations. The following bullet points may help: You have already calculated the amount of sodium bicarbonate in 100 mL In a 100 mL solution of acetic acid at 0.1% there will be 0.1 mL of acetic acid. ● Volume of gas produced The density of acetic acid is 1.05 g/mL. You can use this value to calculate the mass of acetic acid used in the reaction.arrow_forward
- How many moles of W are needed to react with 1.0 mol of Ho in the hypothetic chemical reaction? 3W + 2Ho → 2W3Ho a. 0 b. 1.5 c. 2 d. 3 e. 1arrow_forwardHelp pleasearrow_forwardFor the reaction shown, find the limiting reactant for each of the initial quantities of reactants. express all answers as chemical forumals 2Li(s)+Br2(l)→2LiBr(s) -1 molLi ; 1 molBr2 -1.8 molLi ; 1 molBr2 -2.2 molLi ; 1 molBr2 -arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Expert Answers to Latest Homework Questions
Q: I hope the solution is on paper and clear without the solution using artificial intelligence
Q: I need answer of this question solution general accounting
Q: Kindly help me Accounting question
Q: I hope the solution is on paper and not by artificial intelligence
Q: A reflex klystron operates at the pcak of the n-2 mode. If (V1/Vo)-0.363, find the
efficiency.…
Q: General Accounting Question please correct calculation with explanation for this question
Q: I won't this question answer general Accounting not use ai
Q: Maize company incurs a cost solve this accounting questions
Q: Do fast and step by step calculation with explanation for this general accounting question
Q: Liam owns a nondepreciable capital asset held for investment. If you give correct answer for this…
Q: I need answer of this question solution general accounting
Q: I don't need ai answer general accounting
Q: Part A
Give the IUPAC name and a common name for the following ether:
CH3-CH2-O-CH2-CH2-CH3
Spell…
Q: Give me answer general accounting question
Q: During the current year,a corporation sellls equipment solve this general accounting question
Q: Simmons Enterprises exchanged land and cash of... Please answer the general accounting question
Q: Need answer the accounting question please answer do fast and correct
Q: Please need answer the general accounting question
Q: I hope the solution is on the paper and clear
Q: I hope the solution is on the paper and clear
Q: Provide answer financial accounting