
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:MINDTAP
Q Search this
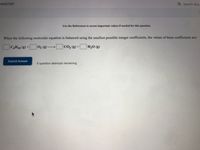
Use the References to access important values if needed for this question.
When the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are:
|C4H10 (g) +
02 () CO2 (g) +|
H2O (g)
>
Submit Answer
5 question attempts remaining
Expert Solution
arrow_forward
Step 1
Balance the chemical reaction using smallest possible integer coefficient
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the reactants and products in a chemical equation, and complete the tables to indicate how many atoms of each type of element are present on either side of the following equations. 2KCO (s) --> 2KCl (s) + O2 (g)arrow_forwardBalance the following reaction. Enter a number in each blank. You must answer numerically, eg "1" not "one". FeBr3 + H2SO4 > Fe2(SO4)3 + HBrarrow_forwardPlease balancearrow_forward
- C6H12O6 O + 6O2 →→ 6CO2 + 6H2O + Energy Determine the limiting reactant and how much of that limiting reactant you would need in order to use up all of the non-limiting reactant. Assume you have 25 grams of glucose and 40 grams of oxygen as reactants for the following photosynthesis reaction: A) Glucose is the limiting reactant; You would need .1388 moles of glucose to use up all of the Oxygen (non-limiting reactant). B Glucose is the limiting reactant; You would need .208 moles of glucose to use up all of the Oxygen (non-limiting reactant). C Oxygen is the limiting reactant; You would need 1.25 moles of Oxygen to use up all of the glucose (non-limiting reactant). D Oxygen is the limiting reactant; You would need .8328 moles of Oxygen to use up all of the glucose (non-limiting reactant).arrow_forward80°F Mostly sunny Stoichiometry can be used to determine the mass (in grams) of a product. O True O False Search H &arrow_forwardPlease balance the following two equationsarrow_forward
- All one questionarrow_forwardFor the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 2 Na(s) + Br₂(g) → 2 NaBr(s) 2 mol Na, 2 mol Br₂ Express your answer as a chemical formula. Part B A chemical reaction does not occur for this question. Submit Request Answer 1.8 mol Na, 1.4 mol Brą Express your answer as a chemical formula. Submit ΑΣΦ Part C A chemical reaction does not occur for this question. Request Answer ΑΣΦ Submit [MD] 2.5 mol Na. 1 mol Brą Express your answer as a chemical formula. Part D ΑΣΦ A chemical reaction does not occur for this question. Request Answer 12.6 mol Na. 6.9 mol Brą Express your answer as a chemical formula. ΑΣΦ ? ? A chemical reaction does not occur for this question.arrow_forwardWithout reference to a table, arrange these reactions according to increasing AS. 1) CH₂(g) + H₂O(g) → CO(g) + 3H₂(g) 2) C(s) + O₂(g) → CO₂(g) 3) H₂O₂(1)→ H₂O(l) + 1/2O₂(g) A) 2<3<1 B) 1<3<2 C) 3 < 1<2 D) 3<2<1 Question 23 of 33 E) 2<1<3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY