
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
YEAR 11 HSC CHEMISTRY

Transcribed Image Text:When calclum chlonde solution is added to sodlum carbonate solution in a beaker, the
mixture quickly becomes cloudy because a precipitate of caldum carbonate foms. Calclum
cartonate is Insoluble in water. The equation for the reaction can be witten as:
Cacz(aq) + NazcO3(aq) +2Naci(aq) + Cacoz(s)
Rewrite this equation as a full lonic equation. Include the relevant physical states.
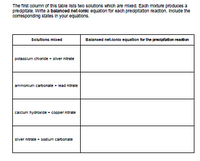
Transcribed Image Text:The first column of this table lists two solutions which are mixed. Each mixture produces a
predpitate. Wirte a balanced net-lonic equation for each precpitation reaction. Include the
corresponding states In your equations.
Solutions mixed
Balanoed net-lonlo equation for the preolpitation reaction
potasalum chloride + sliver nitrate
ammonium carbonate + lead nitrate
calcum hydroxide + copper nitrate
slver nitrate + sodlum carbonate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is green chemistry? Choose the BEST answer. O Chemistry that uses only recycled chemicals. O Chemistry that makes something green in color. O Chemistry that comes from plants or uses plant materials. O Chemistry that uses non-toxic, non-harmful chemicals.arrow_forwardOf the flasks or laboratory instruments that are given, express their function (specify -if they can be used to measure- if their measurements are approximate or relatively exact) Erlenmeyer flask beaker burettearrow_forwardCl₂(g) + 2e Cr₂0 (aq) + 14 H(aq) + 6€¯ O2(g) +4 H(aq) + 4e¯ MnO2(s) + 4H(aq) + 2e¯ 10, (aq) +6H(aq) +5e¯ VO₂+ (aq) + 2H(aq) + e¯ Br₂(1) + 2e NO, (aq) +4 H(aq) + 3e¯ ClO2(g) + e 2 Cl(aq) Ag* (aq) + e Fe3+(aq) + e 2 Cr³+ (aq) + 7 H₂O(1) 1.36 1.33 2 H₂O(1) 1.23 Mn2+(aq) + 2 H₂O(1) 1.21 12(aq) + 3H2O(l) 1.20 VO²+(aq) + H₂O(1) 1.00 2 Br (aq) 1.09 NO(s) + 2 H2O(1) 0.96 CIO₂ (aq) 0.95 Ag(s) 0.80 Fe2+(aq) 0.77 What is the E° cell for the reaction Cr₂O²² + CIO₂ → Cr³+ + CIO₂? a. 2.28 V. b. -2.28 V. c. 0.38 V. d. -0.38 V.arrow_forward
- An electric current of 481.0 mA transports 530. uC of charge. Calculate the time this took. Be sure your answer has the correct unit symbol and 3 significant digits. 0 x10 X H 8 Sarrow_forwardA chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of glycerol, carbon tetrachloride, pentane, tetrahydrofuran, and acetone. The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from her collection of Material Safety Data Sheets (MSDS), the chemist finds the following information: liquid density glycerol -3 1.3 g-cm - 3 1.6 g cm carbon tetrachloride -3 0.63 g·cm pentane tetrahydrofuran -3 0.89 g·cm -3 0.79 g·cm acetone Next, the chemist measures the volume of the unknown liquid as 0.786 L and the mass of the unknown liquid as 991. g. Calculate the density of the liquid. Round your g. cm answer to 3 significant digits. yes Given the data above, is it possible to identify the liquid? no glycerol carbon tetrachloride pentane If it is possible to identify the liquid, do so. tetrahydrofuran acetonearrow_forwardMany common materials that we ingest, though quite safe in reasonable quantities, become toxic when taken in very large doses. A measure of toxicity is the LD50 value (Lethal Dose, 50%). It is the quantity of material, expressed in mg of material per kg of subject-body-weight that, if administered to a population of subjects, would cause 50% of the population to die. The LD50 value for FD&C Red Dye No. 40 is >10,000 mg/kg in rats. Assume that the LD50 value for humans is the same as for rats. Calculate the number of mg of Allura Red present in an 8 fluid ounce glass of the beverage you used in this lab. Assume that the concentration of Allura Red in the beverage is 0.000054 M. The molar mass of Allura Red is 496.42 grams/mol 1 fl oz = 29.5735 mL Do not include units with your answer.arrow_forward
- Suppose some measurements are made on two different homogeneous stones to find out if they are made of the same kind of rock. The mass and volume measurements are listed below. Are the two stones the same type of rock? Why or why not? Show all calculations. Mass Volume Calculations Stone 1 58.0 g 20.0 cm Stone 2 50.1 g 15.0 cm3 21/common/assets/pdfjs/1.0.0.30/web/viewer.ht...ndered-pdf&fullscreen=Dd21-fileviewer-rendered-pdf-dialog&height=746#0arrow_forwardA chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of chloroform, ethanolamine, dimethyl sulfoxide, pentane, and carbon tetrachloride. The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from his collection of Material Safety Data Sheets (MSDS), the chemist finds the following information: liquid chloroform density 1.5 g cm 3 ethanolamine 1.0 g-cm dimethyl sulfoxide 1.1 g cm pentane 0.63 g-cm carbon tetrachloride 1.6 g-cm Next, the chemist measures the volume of the unknown liquid as 0.688 L and the mass of the unknown liquid as 543. g. Calculate the density of the liquid. Round your answer to 3 significant digits. Given the data above, is it possible to identify the liquid? If it is possible to identify the liquid, do so. gcn cm yes no chloroform ethanolamine dimethyl sulfoxide pentane carbon tetrachloride X 5…arrow_forwardThe world population is estimated to be 7.4 x 109. Nauru is the smallest island nation and comprises 1.5 x 10-4% of the world population. If the percentage of left-handed people is approximately 12%, estimate the number of left-handers on the island of Nauru.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY