
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
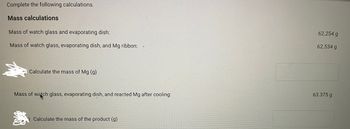
Transcribed Image Text:Complete the following calculations.
Mass calculations
Mass of watch glass and evaporating dish:
Mass of watch glass, evaporating dish, and Mg ribbon:
Calculate the mass of Mg (g)
Mass of watch glass, evaporating dish, and reacted Mg after cooling:
Calculate the mass of the product (g)
62.254 g
62.534 g
63.375 g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1.50 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 180. g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured: product mass carbon dioxide 2.20 g water 0.90 g Use this information to find the molecular formula of X.arrow_forwardA student measured a 6.790 g sample of a hydrated salt. After heating, the mass of anhydrous salt was found to be 4.501 g. Use these data to calculate: a) The mass of water in the hydrated salt.b) The percent by mass of water in the hydrated salt.arrow_forward2.00 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 160. g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured: product mass carbon dioxide 3.30 g water 0.90 g Use this information to find the molecular formula of X.arrow_forward
- A scientist is trying to discover information about an unknown metal in a compound. The formula for the compound is believed to be XBr, where X is the unknown metal. The scientist determined that a 4.720 g sample of this compound contains 4.745 x 10-2 mol Br. Calculate the atomic mass of the unknown metal, X. atomic mass = amu What is the identity of the metal? Provide the name or symbol of the element. metal:arrow_forward1.00 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 26. g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured: product mass carbon dioxide 3.39 g water 0.69 g Use this information to find the molecular formula of X.arrow_forwardInclude a CLAIM: answer the question. For example, this answer is not correct. Include EVIDENCE: Describe what you see in the calculation work that supports your claim. In the answer the wrong conversion factor was used. When calculate mass we..... Include REASONING: Explain WHY you selected that evidence to support your claim. You should have a paragraph.arrow_forward
- If green spheres represent chlorine atoms, yellow-green spheres represent fluorine atoms, white spheres represent hydrogen atoms, and all the molecules are gases, A.write the formula for each of the reactants. Express your answers as chemical formulas separated by a comma B. write the formula for each of the products. Express your answers as chemical formulas separated by a comma.arrow_forwardMolar Mass Representative Particle Mass Number of Substance Number of moles (gimol) (9) Particles Carbon dioxide (CO2) 44.01 molecule 2.5 1.505 x10 Gold (Au) atom 197.00 1 6.02 x10 Glucose (C:H»Os) 180.18 molecule 360.36 1.25x102 Calcium Fluonde (CafFa) 78.08 formula unit 234 24 3 Nitrogen gas (Na) 28.02 mloecule 140.10 3.010 x10arrow_forward2. 5.88 g of hydrated calcium chloride CaCl,•xH,O is treated with excess K2SO4 in a double replacement reaction to yield 5.44 g anhydrous CaSO4 after heating. a) Write down the balanced double replacement reaction. (Refer to question 1a.) b) Calculate the number of moles of anhydrous CaSO4 produced. c) Calculate the number of moles of CaCl,•XH2O used. d) Calculate the molar mass of CaCl2•xH2O. e) Determine the value of x in CaCl,*XH2O.arrow_forward
- A student, Ken, is given a mixture containing two carbonate compounds. The mixture includes MgCO, and (NH, ),CO,. The mixture is 69.14% CO, is by mass. What is the mass percent of MgCO, in the mixture? mass percent of MgCO,: とTOOLS x10 3,438 19 ON A tv MacBook Air DD 80 000 000 F12 F8 F9 F10 F11 F7 ofarrow_forwardCrime scene investigators keep a wide variety of compounds on hand to help with identifying unknown substances they find in the course of their duties. One such investigator, while reorganizing their shelves, has mixed up several small vials and is unsure about the identity of a certain powder. Elemental analysis of the compound reveals that it is 67.31 % carbon, 6.978% hydrogen, 4.617% nitrogen, and 21.10% oxygen by mass. Which of the compounds could the powder be? C17H19NO3C17H19NO3 = morphine, analgesic C17H21NO4C17H21NO4 = cocaine, illicit drug C7H5N3O6C7H5N3O6 = 2,4,6-trinitrotoluene (TNT), commonly used explosive C10H15NC10H15N = methamphetamine, stimulant C4H5N2OC4H5N2O = caffeine, stimulant C11H15NO2C11H15NO2 = 3,4-methylenedioxymethamphetamine (MDMA), illicit drug C21H23NO5C21H23NO5 = heroin, illicit drug C3H6NO3C3H6NO3 = hexamethylene triperoxide diamine (HMTD), commonly used explosivearrow_forward4.33 g sample of a compound consisting of carbon, hydrogen, oxygen, nitrogen, and sulfur was combusted in excess oxygen. This produced 6.38 g CO2 and 2.88 g H2O. A second sample of this compound with a mass of 5.05 g produced 2.70 g SO3. The third sample of this compound with a mass of 6.95 g produced 2.94 g of HNO3. What is the empirical formula of the compound?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY