
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
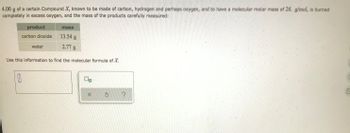
Transcribed Image Text:**Determining the Molecular Formula of Compound X**
In this problem, you are given information about the combustion of a compound known as Compound X, which is composed of carbon, hydrogen, and possibly oxygen. The compound has a molecular molar mass of 26 g/mol. When 4.00 g of Compound X is burned completely in excess oxygen, the masses of the resulting products are carefully measured, as shown in the table below.
| **Product** | **Mass** |
|----------------------|-----------|
| Carbon dioxide (CO₂) | 13.54 g |
| Water (H₂O) | 2.77 g |
Using this information, your goal is to determine the molecular formula of Compound X.
1. **Determine the moles of carbon and hydrogen:**
- From the mass of carbon dioxide (CO₂), calculate the moles of carbon.
- From the mass of water (H₂O), calculate the moles of hydrogen atoms.
2. **Consider the potential presence of oxygen:**
- If Compound X contains oxygen, evaluate its contribution by comparing the total mass of the products to the mass of Compound X.
3. **Calculate the empirical formula:**
- Combine the ratios of moles of each element to find the simplest whole number ratio.
4. **Determine the molecular formula:**
- Use the given molecular molar mass (26 g/mol) to convert the empirical formula to the molecular formula.
You can enter your findings into the provided input box to finalize your answer.
(Note: The molecular formula should be expressed in terms of the subscripts of the elements, indicating the quantity of each element in a molecule of Compound X.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Sssssarrow_forwardThe combustion of gasoline produces carbon dioxide and water. Assume gasoline to be pure octane (C8H18) and calculate how many kilograms of carbon dioxide are added to the atmosphere per 7.8kg of octane burned.arrow_forward7.50 g of a certain Compound X , known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 136./gmol , is burned completely in excess oxygen, and the mass of the products carefully measured:arrow_forward
- Consider the following balanced chemical reaction: HNO3 + 3H2 → NH2OH + 2H2O If 25.0 grams of HNO3 were mixed with 8.70 grams of H2 gas, how many grams of NH2OH would result? Molar mass of HNO3 = 63.01 g/mol Molar mass of NH2OH = 33.03 g/molarrow_forwardUse the References to access Important values If needed for this question. For the following reaction, 5.40 grams of ammonia are allowed to react with 17.1 grams of oxygen gas. ammonia (g) + oxygen (g) nitrogen monoxide (g)+ water (g) What is the maximum amount of nitrogen monoxide that can be formed? What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? Submit Answer Retry Entire Group 2 more group attempts remaining grams grams Previousarrow_forwardConsider the following balanced equation: 2 H2 + O2 → 2 H2OWhat mass of water (H2O) will be collected if 20.0 grams of oxygen gas (O2) are consumed? (Mass of H2O = 18.02 g/mol and Mass of O2 = 32.00 g/mol)arrow_forward
- Liquid hexane CH3CH24CH3 reacts with gaseous oxygen gas O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O . If 3.58g of water is produced from the reaction of 6.03g of hexane and 11.5g of oxygen gas, calculate the percent yield of water.arrow_forwardDuring the decomposition of KClO3, 4.14 grams of oxygen gas are created. How many moles of KClO3 reacted? Report your answer with 3 significant figures. 2 KClO3 → 2 KCl + 3 O2arrow_forwardUse 1 decimal point for all atomic masses. 12.3 g of NCl3(g) are reacted with 0.605 g of H2(g) by the following reaction NCl3(g) + 3H2(g) --> NH3(g) + 3HCl(g) What is the limiting reagent? NCl3(g) H2(g) Based on the limiting reagent, what should the yield of NH3(g) be?arrow_forward
- 9.00g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 60./gmol, is burned completely in excess oxygen, and the mass of the products carefully measured: product mass carbon dioxide 13.20g water 5.40g Use this information to find the molecular formula of X.arrow_forwardFor the reaction shown, calculate how many moles of NO2NO2 form when each amount of reactant completely reacts. 2N2O5(g)→4NO2(g)+O2(g)arrow_forwardThe combustion of propane produces carbon dioxide and steam. C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(g) All of the following statements concerning this reaction are correct EXCEPT a) three molecule of carbon dioxide are formed per one molecule of propane consumed. b) five molecules of oxygen are consumed per one molecule of propane consumed. c) four moles of steam are formed per five moles of oxygen consumed. d) the combined mass of reactants consumed equals the mass of products formed. e) three grams of carbon dioxide are formed per five grams of oxygen consumed.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY