
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Include a CLAIM: answer the question. For example, this answer is not correct.
Include EVIDENCE: Describe what you see in the calculation work that supports your claim.
In the answer the wrong conversion factor was used. When calculate mass we.....
Include REASONING: Explain WHY you selected that evidence to support your claim.
You should have a paragraph.

Transcribed Image Text:In class, students were given the following problem:
"Aluminum satellite dishes resist corrosion because aluminum reacts with oxygen gas (0₂) to form a
coating of aluminum oxide (Al2O3) according to the following chemical equation:
4Al(s) + 302(g) →2A/203(s)
What is the mass of 9.45 moles of Al 203?
After correctly solving for the molar mass of Al2O3, Jacob set up the following conversion:
1 mole AL, Q
9.45 moles Al₂O3× 102.2 grams of Al, 0,
Is this answer correct?
= 0.0926 grams of Al2O3
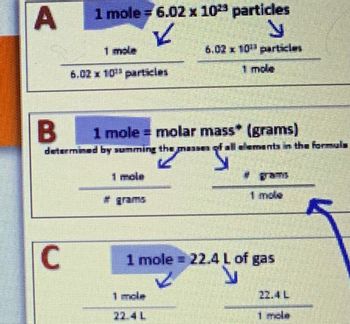
Transcribed Image Text:A
1 mole = 6.02 x 10²³ particles
✓
N
6.02 x 10 particles
1 mole
C
6.02 x 10¹ particles
B 1 mole = molar mass* (grams)
"S
determined by summing the ma 1 of all elements in the formula
1 mole = 22.4 L of gas
Inde
7241
22.4 L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A sample of Cl₂ gas @STP has a volume of 13L. How much does it weigh? Use only the following units • atoms or particles or molecules • mol Make sure to use the superscripts above for Avogadro's number. There should be NO spaces in any box. 12345678910 superscripts Given (numberunit): type your answer... Wanted (unit) type your answer... Is the GIVEN or WANTED g? If so, calculate the molar mass and type it into the box, if it doesn't, type skip in this box. type your answer... type your answer... H type your answer... type your answer... type your answer... type your answer...arrow_forwardThe formula for alum has 1 Al. How many units alum could be made from 7 Al atoms? Answer with a number.arrow_forwardWhich of the following is incorrect? A. Fluorine will always have an oxidation number of -1 in all its compounds B. Oxidation numbers are always integers C. In a neutral compound, the sum of the oxidation numbers of all the atoms must be equal to zero D. Halogens usually have an oxidation number of -1 when bonded with other elements, except when bonded with oxygen. Which of the following is correct? A. Oxidation numbers are always integers B. Alkaline earth metals have an oxidation number of +1 in their compounds C. The oxidation number of hydrogen is +1 when bonded with non-metals, -1 when bonded with metals D. Alkali metals have an oxidation number of +2 in their compounds.arrow_forward
- = AA ALEKS - Andrew Herrera - Learn O CHEMICAL REACTIONS Calculating and using the molar mas... Explanation Check www-awa.aleks.com b Register for bartleby | bartleby A chemist measures the amount of chlorine gas produced during an experiment. He finds that 11.0 g of chlorine gas is produced. Calculate the number of moles of chlorine gas produced. Round your answer to 3 significant digits. 0₁ mol x10 G english soanish - Google Search 3/5 : 202 Moraw Mill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardof 10 4. Write the following balanced equations. • Solid iron(III) sulfide reacts with gaseous hydrogen chloride. • Magnesium combines with nitrogen. Zinc reacts with silver chloride. C Text Predictions: On 2268 words Type here to search Focusarrow_forward(Mass of crucible and cover is 43.92 g. The mass of crucible, cover, and magnesium is 44.62g. Mass of crucible, cover, and magnesium oxide is 44.99g) a) Mass of Mg reacted b) Mass of magnesium oxide formed c) Mass of oxygen in magnesium oxidearrow_forward
- Use the following information to determine the mass of chlorine combined with the magnesium to two decimal places for the compound formed. Mass crucible 35.1 Mass crucible and magnesium 38.92 Mass crucible and magnesium chloride compound 40.6arrow_forwardA mixture of 20.0 g of P and 78.8 g of Cl₂ reacts completely to form PC13 and PC15 as the only products. Part A Find the mass of PC13 that forms. Express your answer with the appropriate units. mpcl3 38.2 = μĂ g www Submit Previous Answers Request Answer ? X Incorrect; Try Again; One attempt remainingarrow_forwardBlank #1) Calculate the mass of 1.98 moles of Li₂SO3 in grams. Report you answer with three sig.figs. Blank #2) Show your conversion factor set-up in the second blank. Use this format: 200 minutes X (1 hour/60 minutes) X (1 day/24 hours) Blank #1 Blank #2 Question 7 (4 points) Listen Blank #1) One tablet of aspirin, C9H8O4, contains 0.0212 moles. How many grams is this? Report your answer with three sig.figs. Blank #2) Show your conversion factor set-up in the second blank. Use this format: 200 minutes X (1 hour/60 minutes) X (1 day/24 hours) Blank # 1 Blank # 2 A Aarrow_forward
- 11. When the equation 3 _H2 +_ N2 - _ NH3 7 is correctly balanced using the smallest whole numbers, what will be the sum of all the coefficients? 6 12 CLEAR ALLarrow_forwardA sample of carbon weighting 1.48 g was burned in an excess of air. The mass of carbon dioxide, the sole product was 5.42 g. In a second experiment 11.62 g of carbon dioxide was obtained. What mass of carbon was burned in the second experiment?arrow_forward4. A student obtains the following data. 25.2246 g mass of clean, dry and empty crucible 25.3372 g mass of crucible with magnesium strip 25.3965 g mass of crucible with the oxide product Determine the empirical formula that this student should have obtained. (Note: this was actual data from a student, so the empirical formula is not what we might have predicted!)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY