
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
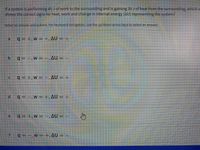
Transcribed Image Text:ifa system is performing 45 Jof work to the surrounding and is gaining 30 J of heat from the surrounding, which o
shows the correct signs for heat, work and change in internal energy (AU) representing the system?
Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer.
q = +, w = +, AU = +
q = -,W = ,AU = –
q= +,w - -, AU = +
q=-,w= +, AU = +
q =+,w= =,AU = –
q= -, w =+,AU = –
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- ||| Solving a basic calorimetry problem A sample of polystyrene, which has a specific heat capacity of 1.880 J.g¹.C¹, is put into a calorimeter (see sketch at right) that insulated contains 200.0 g of water. The polystyrene sample starts off at 92.7 °C and the temperature of the water starts off at 15.0 °C. When the temperature of the water stops changing it's 23.1 °C. The pressure remains constant at 1 atm. container Calculate the mass of the polystyrene sample. Be sure your answer is rounded to 2 significant digits. Me g x10 X thermometer. 5 water sample a calorimeterarrow_forwardA chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 6.30 kg of water at 22.3 °C. During the reaction 64.3 kJ of heat flows out of the flask and into the bath. - 1 -1 Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18 J'g *K '. Ве sure your answer has the correct number of significant digits. dloarrow_forwardI need some help solving this questionarrow_forward
- o-phthalic acid, C8H6O4, is sometimes used as a calorimetric standard. Its heat ofcombustion is - 3.224 × 103 kJ/mol C8H6O4. From the following data determine theheat capacity of a bomb calorimeter assembly (i.e., of the bomb, water, stirrer,thermometer, wires, etc.)mass of o-phthalic acid burned: 1.078 ginitial calorimeter temperature 24.96 °Cfinal calorimeter temperature 30.76 °Carrow_forwardThe system releases 121 J of energy while 113 J of work was done on it Calculate the change in energy for the following processes and determine if it is endothermic or exothermic. -234 J, exothermic +8 J; exothermic +8 J, endothermic +234 J, exothermic -8 J; endothermic -8 J, exothermic -234 J; endothermic +234 J; endothermicarrow_forward[Review Topics] [References] Use the References to access important values if needed for this question. Given the standard enthalpy changes for the following two reactions: (1) 2Pb(s) + O2(g) → 2PbO(s) (2) Pb(s) + Cl2 (g) → PbCl₂ (s) AH° what is the standard enthalpy change for the reaction: (3) 2PbCl2 (s) + O2(g) → 2PbO(s) + 2Cl2 (g) AH° = ? Submit Answer AH-434.6 kJ AH = -359.4 kJ kJ Retry Entire Group 9 more group attempts remainingarrow_forward
- Which of the following signs on q and w represent a system that is doing work on the surroundings, as well as losing heat to the surroundings? q=-, w = - q=+, W = + q=, W = + q=+, w = - O None of these represent the system referenced above.arrow_forwarduestion 22 of 41 > If a system has 225 kcal of work done to it, and releases 5.00 × 10² kJ of heat into its surroundings, what is the change in internal energy (AE or AU) of the system? kJ AE = étv MacBook Air DII 80 888 F12 F10 F11 F7 FB F9arrow_forwardO Thermochemistry Using specific heat capacity to find heat 0/5 Jaqu Calculate the energy required to heat 474.0 g of cyclohexane from 21.1 °C to 37.1 °C. Assume the specific heat capacity of cyclohexane under these conditions is 1.85 J.g¹K -1 . Round your answer to 3 significant digits. 0 ロ・ロ X μ G ㅁㅁarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY