
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
None
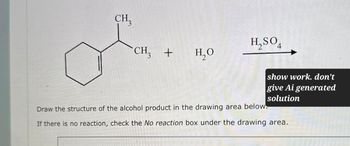
Transcribed Image Text:CH₂
H₂SO4
CH3 +
H₂O
Draw the structure of the alcohol product in the drawing area below.
show work. don't
give Ai generated
solution
If there is no reaction, check the No reaction box under the drawing area.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- Iron(III) Chloride Test Saved BIIIU X2 | X² | → Ix Normal Alcohol FeCl3 test color Ethanol 2-Propanol 2-Methyl-2-propanol Cyclohexanol Phenol N/A Unknown ll !!arrow_forwardH. An ester is produced by the reaction of an alcohol with a carboxylic acid. For each ester below, underline the alcohol part and circle the carboxylic acid part. Then, name each ester. CHỊCH,CH,CH,CH,-O-C-CH,CH,CH, H CH₂ CH₂ CH 1 CH3arrow_forwardBr 1. Mgº, Ether 2. CO₂ 3. H3O+ 1. HBr, H₂O2, hv 2. Mgº, Ether 3. H 4. H3O+ (mild) 5. PCCarrow_forward
- Match the following reactions as to which one would give the best, middle, and worst conversion. oh CI HO, NH2 Harrow_forwardComplete the given reactions. Add hydrogen atoms and charges to the appropriate atoms. Reaction A H₂C-CH₂-C -NCH, + NaOH H H₂C-CH₂-C-N-CH₂ + HCI H heat 2 heat Incorrect Reaction B OH Y H₂C 0 Na H₂N CH₂ H₂C OH H₂N CH₂arrow_forwardPlease help! I need to know how to draw the full chemical structure of isoproyl red. *I drew the structure of isopropyl alcohol (second pic) but was told this was incorrect and that the chemical structure needs to show a combination of methyl red and isopropyl alcohol.arrow_forward
- 12.arrow_forwardH₂N. Structure 1 ОН H₂N .OH Structure 2 HaN. Reaction Bank: Reaction A: 'N' لز (H3CC" Reaction B: DCCD, HCI Reaction C: CF3CO2H ہ C(CH3)3 OHarrow_forwardNBS ·Br Mg, ether> CO2 9. Determine whether the following reactions are flawed. Put "Yes" on the line for flawed reactions and for reactions that are not flawed (3 points). MeO COME LIAIH4 HgSO4 H3O EtOH H₂O 7 O EtQ OEt 667 MeOarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY