
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
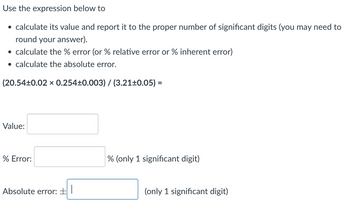
Transcribed Image Text:Use the expression below to
⚫ calculate its value and report it to the proper number of significant digits (you may need to
round your answer).
⚫ calculate the % error (or % relative error or % inherent error)
⚫ calculate the absolute error.
(20.54±0.02 × 0.254±0.003) / (3.21±0.05) =
Value:
% Error:
Absolute error: ± |
% (only 1 significant digit)
(only 1 significant digit)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Calculate the value and report to the proper number of significant digits (you may need to round your answer). Calculate the % error (or % relative error or % inherent error) and the absolute error. (20.54+0.02 × 0.254±0.003) / (3.21±0.05) = Value: % Error: 0.1 absolute error: +3 % (only 1 significant digit) (only 1 significant digit)arrow_forwardUse the expression below to ⚫ calculate its value and report it to the proper number of significant digits (you may need to round your answer). ⚫ calculate the % error (or % relative error or % inherent error) ⚫ calculate the absolute error. (30.078±0.003) - (20.174±0.001) + (9.813±0.005) = Value: % Error: absolute error: ± % (only 1 significant digit) (only 1 significant digit)arrow_forwardSolve the value of x in the expression and express your answer in correct significant figures log ( [3.0 x 10 -2][2.0 x 10 4] / (2.0 x]3 ) = 1.347 The value of x, in correct significant figures, isarrow_forward
- 1000 Incorrect! But, you can still earn a point by determining the uncertainty value for the correct measurement. 900 883 ± mL 800 SUBMIT UNCERTAINTY 200 100arrow_forwardThe relative error for the following data below is: 39.63, 39.61, 39.05, 39.08 (the true accepted value= 40.00) Please fill in the space with a numerical value with two digits, without any unit and including sign (-) or (+).arrow_forwardPerform the calculations and determine the absolute and percent relative uncertainty. Express each answer with the correct number of significant figures. To avoid rounding errors, do not round your answers until the very end of your calculations. [9.8 (±0.3) — 2.31 (±0.01)] 8.5 (+0.6) = absolute uncertainty: + 0.76 Incorrect percent relative uncertainty: 0.88 + 86.36 Incorrect 9.2 (+0.4) × ([5.4 (±0.3) × 10−³] + [5.6 (±0.1) × 10-³]) = Incorrect %arrow_forward
- Please answer.arrow_forwardFind the difference between the value of x2 and the sum of x1 plus x3.; let x4 be equivalent to the sum of x1 and x3 and let x5 be the difference between x2 and x5. This calculation scheme is shown below. Make notes about any similarities or differences between the values in your notes. x4 = x1 + x3 x5 = x2 – x4 Data Analyis This section will include all data collected during the lab. Thermochemical Data Tinitial (°C) Tfinal (°C) ΔT (°C) moles NaOH qreaction (kJ) ΔHrxn Reaction 1 25.0 30.3 +5.3 0.025 -1.11 -44.4 Reaction 2 25.0 37.0 +12.0 0.025 -2.51 -100.4 Reaction 3 25.0 31.7 +6.7 0.025 -1.40 -56.1 Reaction 1: NaOH(s) → Na+(aq) + OH-(aq) + x1 kJ 1g /39.977g/mol = 0.025 moles Moles NaOH = 0.025 qsolution = (4.184 J/g °C) (50.0g) (30.3°C -25.0°C) = -1108.76 J/ 1000 qreaction (kJ) = -1.11 kJ ΔH = -1.11 kJ/ 0.025 moles ΔHrxn = -44.4 kJ/mol…arrow_forwardWhat is the smallest mass that we can measure on an analytical balance that has a tolerance of ±0.1 mg, if the relative error must be less than 0.1%?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
