
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
HELP NOW PLEASE URGENT
Transcribed Image Text:ns <
+1421 pts
/3400
Hide
on.
HP
ch
ces.
< Question 17 of 34
>
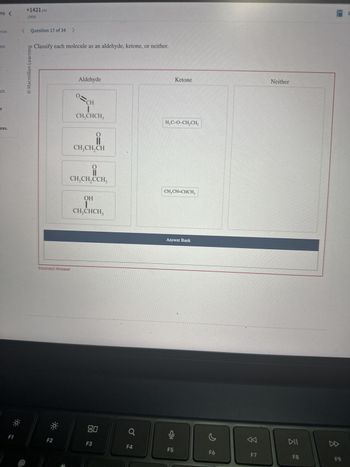
Classify each molecule as an aldehyde, ketone, or neither.
© Macmillan Learning
Ketone
Neither
Aldehyde
0=
=CH
CH,CHCH3
ii
CH3CH2CH
i
CH3CH2CCH3
OH
CH3CHCH3
H₁C-O-CH2CH3
CH3CH=CHCH3
Incorrect Answer
Answer Bank
80
Ơ
<
ད
DII
分
F1
F2
F3
F4
F5
F6
F7
F8
F9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- HO HO. OH base CI EIO- C-OEt epichlorohydrin OH NaOEt 2,6-dihydroxy- acetophenone *Na O2C. .CO2 Na* NaOH, H2O II OH Cromolyn sodium Cromolyn sodium, developed during the 1960s, has been used to prevent allergic reactions primarily affecting the lungs, as, for example, exercised- induced emphysema. It is thought to block the release of histamine, which prevents the sequence of events leading to swelling, itching, and constriction of bronchial tubes. v Cromolyn sodium is synthesized in the above series of steps. rch 31% 11:24 P 4/20/20-arrow_forwardReaction C Select Draw Rings More |||||| C 0 N H 0 || H H₂C C-C I heat H₂C CH–COOH +CH,CH(CH,)NH, H₂C- CH₂ H₂C J Q2Q Draw the amide formed when isopropylamine (CH₂CH(CH³)NH₂) (CH3CH(CH3)NH₂) is heated with each carboxylic acid. Include all hydrogen atoms. What did I do wrong? N HC- Erase CH₂arrow_forward2 SUPERMARKET23 4 Essay Writing Ser. G calculator - Googl. A Gflights b BATERBLY C CHEGG > KATAPULK CUBA O Maps a AMAZON A Translate Question 6 of 21 Which of the following best describes why only small aldehydes and ketones are soluble in water? A) low molar mass aldehydes and ketones can hydrogen bond through the carbonyl oxygen to water. B) High molar mass aldehydes and ketones have boiling points that are too high to be soluble in water. C) low molar mass aldehydes and ketones hydrogen bond with one another to be soluble in water. D) low molar mass aldehydes and ketones have low boiling points that enhance solubility in water. MacBook Pro 23 2 3 4 7 8 W E R Y F G H. J K Tarrow_forward
- TO 23 #F ** ## 4 .. ||| V Classify each of the molecules below. A B 4 CH3C=C NH₂2 primary amine Osecondary amine O tertiary amine Explanation G Identifying prinary, secondary, and tertiary amines A $ W S not an amine at all MB 4 # @ #D S P D MA C D *** # D # R # *40 O M PRE 6 19 # . PP G 4 CO @ 25 * 4 10 10 # ♥ a # B sh • Check B B => R W # B RS 9 Ra 00 • a B # S 66 D W W PR ** 19 S # 4 4 BA M @ D #** 4 68 # $ D → * ** @ 48 4 9 S B S a #4 NG D B 55 # 46 3 S 62 4 TO 0 # # 9 4 D B 6 19 4 C 99 6 O primary amine O secondary amine O tertiary amine O not an amine at all P C B . 10 S 4 4 * 4 CH3 —CH=CH—NH2 S ## S 9 B B D Ⓡ 46 9 12 . O 16 S W 4 D # a 0 W #4 D D 1 @ 4 d ♥ 30. D # @ a S 12 #D $ B ** * B D S B 00 # 99 * W e A W 1 W S 9 A D * s @ 10 6 66 19 S P P 14 * 0 a 4 A 16 糖 U a V # # A ** DO E C 24 SP ** N $ B a **** @G S D # 4 S -A W C NE # SOP * ## @A 44 • B S * 15 B $ L ** * # W A Or C D # ** 4. $ A 68 B ** $3 RE B O 6 C $ * O * # @@ #D De A 16 8 W N #0 3 @a *** W 慈 B 4 G # ▶ 10 4 BO $…arrow_forward0 0 carboxylic acid CH3CH,CCH2CH3 ester OCH2CH3 anhydride amide CH3CH2CH2CH2CH,COH none of the above CH,CH2CH2CH2CH2CNH2 Cengage Learning | Cengage Technical Support MacBook Airarrow_forwardtaken in order to gor the product Please explain the mechanisms/stepsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY